 In order for the cells that make up an organism such as you to work in harmony, they must be able to communicate and collect information about their environment. The idea that our cell receptors—cellular signal receivers—may be blocked and unable to function normally is a current theme in food blogging, health guru lectures, and essential oil sales hype. “Experts” instruct us in their method of “cleaning” the receptor in order to restore “proper signaling.” One such ‘expert’ is David Stewart, who claims that:
In order for the cells that make up an organism such as you to work in harmony, they must be able to communicate and collect information about their environment. The idea that our cell receptors—cellular signal receivers—may be blocked and unable to function normally is a current theme in food blogging, health guru lectures, and essential oil sales hype. “Experts” instruct us in their method of “cleaning” the receptor in order to restore “proper signaling.” One such ‘expert’ is David Stewart, who claims that:
“Drugs and oils work in opposite ways. Drugs toxify. Oils detoxify. Drugs clog and confuse receptor sites. Oils clean receptor sites. Drugs are designed to send misinformation to cells or block certain receptor sites in order to trick the body into giving up symptoms.” (Stewart 2005). He also claims that phenylpropanoids, a class of chemicals found in some essential oils, can clean receptor sites on the surfaces of cells (Stewart 2007). These claims are not supported by science, and they are inconsistent with what we know about receptor biology.
Receptor-ligand binding
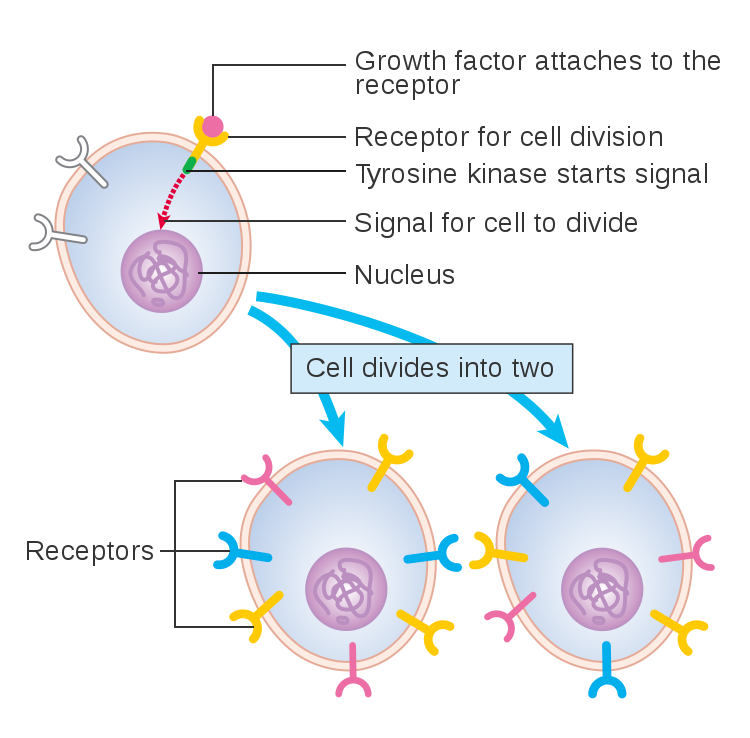
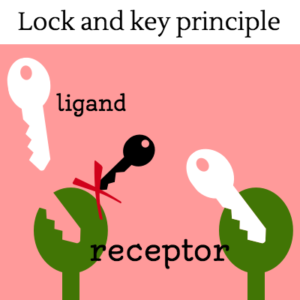 Cells must be able to determine the best time to grow, divide, or undergo any number of changes. This partly takes place through gene regulation, which is affected by a number of factors, including signals that are received at the cell membrane. These extracellular signals are called ligands, which may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules or nutrients. Ligands bind to special cellular protrusions called cell receptors. Cell receptors are complex proteins uniquely shaped to interlock with their corresponding ligand. A common analogy for receptors and ligands is that of locks and keys. When a ligand binds to a receptor, this causes a cascading chemical change through the cell membrane and into the cell.
Cells must be able to determine the best time to grow, divide, or undergo any number of changes. This partly takes place through gene regulation, which is affected by a number of factors, including signals that are received at the cell membrane. These extracellular signals are called ligands, which may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules or nutrients. Ligands bind to special cellular protrusions called cell receptors. Cell receptors are complex proteins uniquely shaped to interlock with their corresponding ligand. A common analogy for receptors and ligands is that of locks and keys. When a ligand binds to a receptor, this causes a cascading chemical change through the cell membrane and into the cell.
Once a ligand binds to a receptor, a protein called ubiquitin binds the ligand-receptor to this complex, which marks it for degradation by lysosomes in the interior of the cell (Jentsch et al 2004, Goh and Sorkin 2013). The rate of degradation is variable and in some cases a ligand may be removed from the complex, making the receptor available or “open” again. This allows the receptor to be recycled back to the cell surface, and it is available for the next round of ligand binding (Huang Fu and Fuchs 2010). Regardless, cell receptors do not generally remain bound to their ligands at the cell membrane. As new receptors are consistently generated or recycled, unbound receptors found at the cell surface would be available for ligand binding (Goh and Sorkin 2013). Because of this biological mechanism, the idea of “blocked receptors” remaining at the cell surface is not likely nor logical. Even when cells mutate, the result is not usually “blocked” receptors.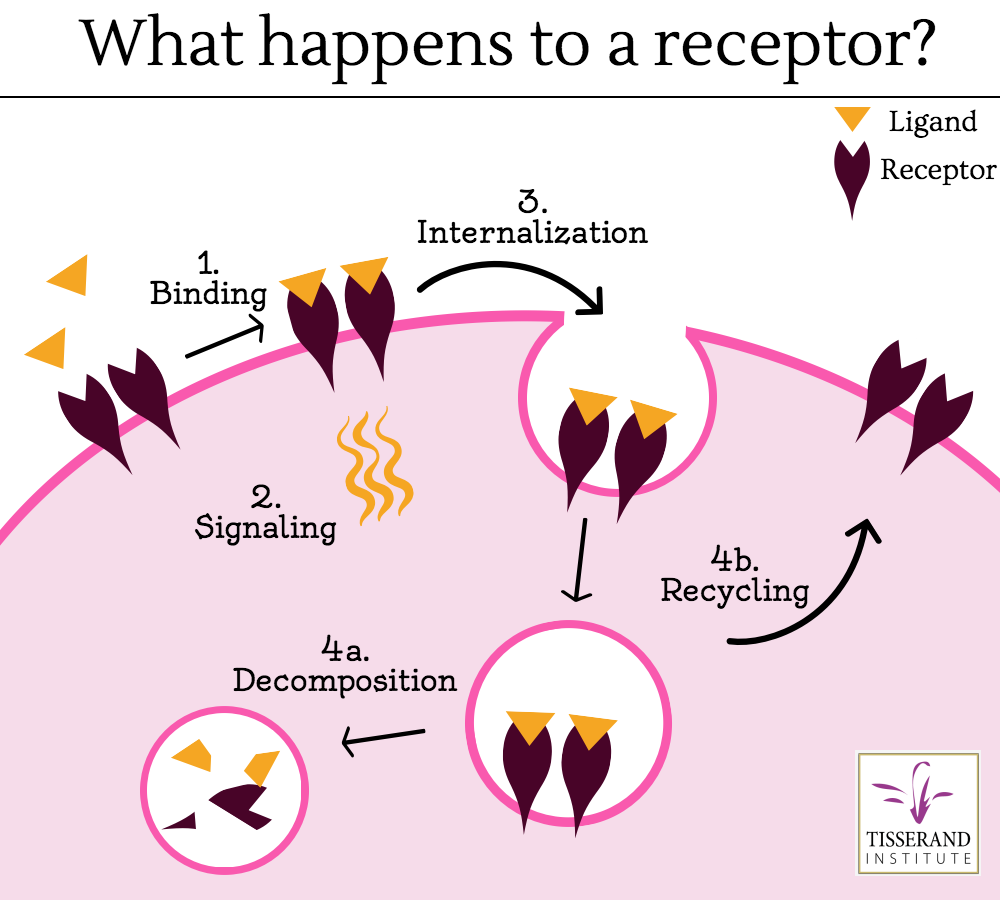
Mutations and dysfunctions in cellular receptors
Mutations to cell receptors do occur, and in the case of cancer, mutation of the receptor is an established mechanism that causes signaling dysfunction. One type of epidermal growth factor receptor, ErbB, can be mutated in patients with a type of blood cancer called erythroleukemia. In these patients, the growth factor binding site in the receptor is truncated such that ligand binding will not occur. These cancer cells are able to activate the resulting signaling cascades without the presence of the growth factor, and in turn, proliferate uncontrollably (Weinberg 2007). In other instances, truncation of the receptor could result in silencing, making the cell unable to react to its environment. The proteins that make up the receptor binding site are altered and cannot accept or be activated by the proper ligand. In this way, the concept of a “blocked receptor” is not applicable, and “cleaning the receptor” would not remedy the dysfunction.
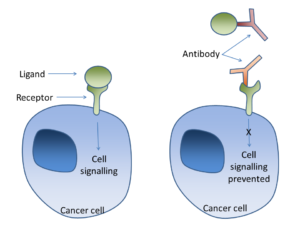
Cell signalling can occur through a ligand binding to a receptor on the cancer cell surface. Antibodies can bind to the ligand (as in bevacizumab-VEGF) or to the receptor (Trastuzumab-Her2/neu) in order to prevent this signalling.
In general, cells do not produce both a ligand and its receptor. Instead, ligands are released by one type of cell, and another cell binds the ligand. However, some of the most “successful” cancers set up an autocrine feedback loop, meaning they produce the ligand and the receptors. Essentially fueling their own growth, they eliminate any regulation of their growth. Cancers such as Kaposi’s sarcoma, which is a cancer of endothelial cells, gain the ability to manufacture PDGF, TGF-beta, IGF1, Ang2, CC18/14, CXCL11, endothelin, and their associated cell receptors. In addition, Kaposi’s sarcoma cells are able to manufacture two herpes-8 ligands that bind receptors normally found in endothelial cells. The presence of these mutations negatively affects patient survival. However, while these cancer cells have an abnormal expression of ligands and receptors, the ligand binding and signaling is normal, and hence, the premise of “blocked receptors” leading to disease is not applicable.
Some cancers overexpress receptors such that the number of cell receptors at the cell membrane is increased relative to normal cells. In the presence of a ligand, these cells would then have an exaggerated reaction to their ligands. Breast cancers often exhibit mutations in the HER2/ErbB2 receptor gene sequence, or overexpression of the gene, which results in an excess of receptors (Brennan et al 2000, Weinberg 2007). “HER2 positive” refers to the presence of the HER2 receptor (diagnosed by histopathology), or gene sequence mutations (diagnosed by DNA-based tests) (Perez et al 2014).
Medications have been developed to target mutated HER2 receptors, in order to inhibit the receptor function (Figure 4). Drugs such as Herceptin, Tykerb, Kadcycla, and Perjeta are compounds that bind the HER2 receptor to block signaling and also flag the cancer for destruction by the immune system (Segovia-Mendoza et al 2015). Blocking the receptor from binding to growth factors is essential to treat this cancer. In a sense, these medications do “send misinformation to cells or block certain receptor sites” (David Stewart 2005). For HER2 positive cancer cells, silencing abnormal cell signaling is a desired result. Blocking the receptor and targeting aberrant cells for destruction is an established mechanism for disease treatment. On the contrary, “cleaning” the receptor of the treatment drug ligand would allow the cancer to continue growing.
Phenylpropanoids: inhibiting cell receptors intentionally
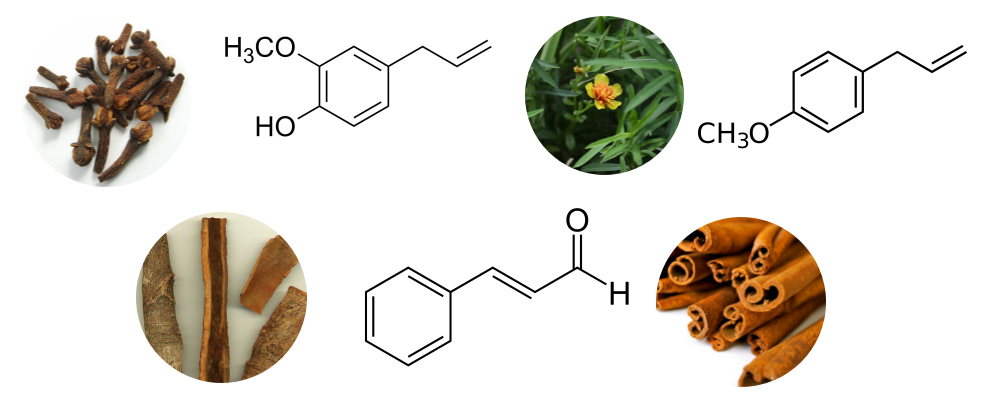
Phenylpropanoids are organic compounds synthesized by plants, fungi, and bacteria from two aromatic amino acids–phenylalanine and tyrosine (Vogt 2010). In plants, these compounds act as a defense mechanism against disease-causing microbes and herbivores (Vogt 2010). Essential oils distilled from clove, cinnamon, cassia, and tarragon contain phenylpropanoids such as eugenol (Figure 5), estragole (Figure 6), safrole and cinnamaldehyde. In essential oils, these compounds have been ascribed antimicrobial effects (Bowles 2004). However, they are also some of the more hazardous chemical constituents. Safrole and estragole are considered to be carcinogens, while eugenol and cinnamaldehyde are potential skin allergens (Tisserand & Young 2014).
Some phenylpropanoids do interact with some cell receptors. Eugenol has been shown to inhibit gamma aminobutyric acid (GABA) receptors, a type of selective ion channel. GABAA receptors in the brain are responsible for fast inhibitory synaptic transmission, which is involved in symptoms of pain. Eugenol is considered an agonist for two other ion channel receptors: TRPV3 (a warmth sensitive receptor) and TRPV1 (an irritant sensitive receptor) (Klein et al 2014, Meents et al 2016). In effect, application of eugenol, which is widely used in dentistry, is effective for pain relief and inflammation because it interacts with cell receptors (Klein et al 2014, Lee et al 2015). It is thought that eugenol directly binds to the receptors, but direct evidence of eugenol binding as a ligand is lacking. However, phenylpropanoids likely inhibit or activate signaling cascades, much like endogenous ligands, rather than by displacing ligands from “blocked receptors”.
Conclusions
Cell receptors are an important part of the communication system in organisms. Ligands such as growth factors bind receptors, and open ion channels or trigger signaling cascades. This system allows the cell to determine which genes need to be active, which proteins should be synthesized, and whether the cell should divide or move. Dysfunction in ligand-receptor interactions and receptor activation is associated with disease, and treatments often seek to eliminate this dysfunction.
Cancer cells also regulate their growth and gene expression based on extracellular signals, but are able to manipulate the cell’s own machinery to drive growth and proliferation to achieve immortality. Whether cancer cells increase the numbers of receptors at the cell surface, eliminate the degradation of bound receptors, or mutate receptors to function without ligand binding, application of phenylpropanoid-containing essential oils will not unblock receptors. Even if this was possible, making a receptor available for ligand binding would likely make the cancer cell more receptive to available ligands, which is not desirable. In this way, the logic of needing to clean receptors to cure disease is faulty. Claiming that drugs send misinformation to cells is likewise false. Although many treatments seek to trigger apoptosis or change gene regulation within the cell, these treatments are designed to eliminate faulty function in diseased cells, which have been able to override their normal function.
Are the claims by David Stewart that “Drugs clog and confuse receptor sites. Oils clean receptor sites. Drugs are designed to send misinformation to cells or block certain receptor sites in order to trick the body into giving up symptoms” true? The short answer is no – these claims are not true. The longer answer is that ligand binding at receptors is not just an issue of function or dysfunction. It’s not simply that ligand binding is good, and not binding is bad – the interaction is variable, and nuanced. Sometimes, for example, the ligand binds but fails to activate the receptor. There is evidence that phenylpropanoids found in essential oils of clove, cinnamon, cassia, and tarragon interact with some cell receptors. However, the action of this binding leads to inhibition or activation of receptors, not the “cleaning” of a bound receptor.
Claims that cell surface receptors need to be stripped by essential oil constituents are not supported by the research. Receptors do not universally need to be “stripped” nor have essential oil constituents been shown to universally “strip” ligands from receptors. Although the science that refutes these ideas is not simple, Stewart’s and others’ claims along with the hype about clogged receptors are simply wrong.
Used terms |
Definition |
|---|---|
| Ang2 | angiopoietin 2 |
| Antioxidant | molecule that inhibits oxidation of other molecules |
| Autocrine | ligand and receptor are produced by the same cell |
| Carcinogen | substance that causes cancer |
| CC18/14 | a type of chemical signal |
| Cell membrane | bilayer membrane that acts as a barrier to the extracellular space |
| Cell receptor | binding site for ligands; performs a biological function in complex with its ligand |
| CXCL11 | a type of chemical signal |
| Detoxification | removal of toxic substances from an organism |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| Endogenous | originate within an organism |
| Endothelin | a vasoconstricting peptide |
| Enzyme-linked receptor | a receptor which undergoes enzymatic changes upon activation, in order to activate signaling cascades |
| Extracellular | outside a cell |
| GABA | gamma aminobutyric acid |
| G protein receptor | a receptor which undergoes conformational change upon activation, in order to bind G proteins and guanosine di- or tri-phosphate to activate signaling pathways |
| HER2/ErbB2 | epidermal growth factor 2 |
| IGF1 | insulin-like growth factor 1 |
| Intracellular | inside a cell |
| Ion channel receptor | a receptor which opens upon activation to allow passage of ions |
| Ions | charged atoms |
| Kit | an oncogenic protein and RTK |
| Ligand | a substance that binds to another structure (in this case, receptors) as a complex, to perform a biological function |
| Lysosome | a cellular organelle involved in degradation |
| PDGFR | platelet derived growth factor receptor |
| Phenylalanine | an amino acid with the chemical formula C9H11NO2 |
| Phenylpropanoid | aromatic compound comprising a 6-carbon ring and a 3-carbon tail |
| Proliferation | growth and division of cells |
| Propyl | 3-carbon propene group |
| Receptor tyrosine kinase | a type of enzyme-linked receptor that binds growth factors. |
| Shikimate pathway | biosynthetic pathway that produces phenylalanine, tyrosine, and tryptophan |
| TGF-beta | transforming growth factor beta |
| TRPV1 | an ion channel receptor involved in thermosensitivity |
| TRPV3 | an ion channel receptor involved in thermosensitivity |
| Tyrosine | an amino acid with the chemical formula C9H11NO3 |
| Ubiquitin | a protein whose binding marks a protein/protein complex for degradation by lysosomes |
References
Stewart, D. (2007). Healing oils of the Bible. 1st ed. Marble Hill, MO, Care Publications.
Tisserand, R. and Young, R. (2014). Essential oil safety. 2nd ed. Edinburgh, Churchill Livingstone.
Vogt, T. (2010). Phenylpropanoid biosynthesis. Molecular Plant, 3(1), 2-20.
Weinberg, R. (2007). The biology of cancer. 1st ed. Garland Science



THANK YOU Shannon and Robert! This is one of the most important articles in the last few years. Good information might hopefully clean the receptors of misinformed victims of dollar-driven and religious-like marketing campaigns for essential oils.Thank you! [[[I will cite/translate a few lines and share this valuable text on my well read German language blog plus the connected social media]]]