There are several boswellic acids (BAs) found in some Frankincense species (notably Boswellia serrata and B. sacra), and the BAs are credited with possessing, among others, antitumoral properties, with 11-keto-β-boswellic acid being one of the most useful therapeutically (Roy et al 2019). However, whether or not Frankincense essential oils contain BAs has been a discussion point for more than 20 years, with people, including myself, arguing either that they are present in the essential oil, or that they are not.
One reason for the controversy has been the claim that Frankincense oil is anti-carcinogenic because it contains BAs, and yet these are never seen in analyses of Frankincense oils (for example, Mertens et al 2009, Niebler & Buettner 2010, Ojha et al 2022, Van Vurren et al 2010). A counter-argument has been that because BAs are non-volatile they will not show up with regular gas chromatography-mass spectroscopy (GC-MS) analysis, and you need to use high-performance liquid chromatography (HPLC) which detects non-volatiles as well as volatiles. However, if BAs are not volatile, how could they be found in an essential oil? Surely essential oils only contain volatile compounds?
Let’s look at the evidence!
Can distilled oils contain non-volatile compounds?
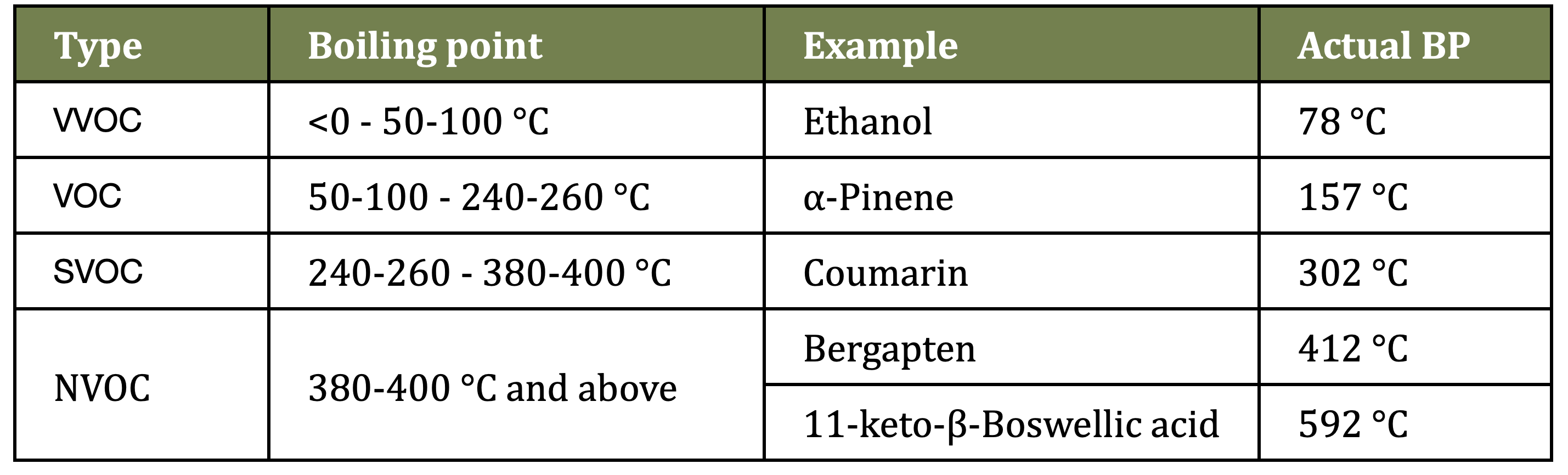
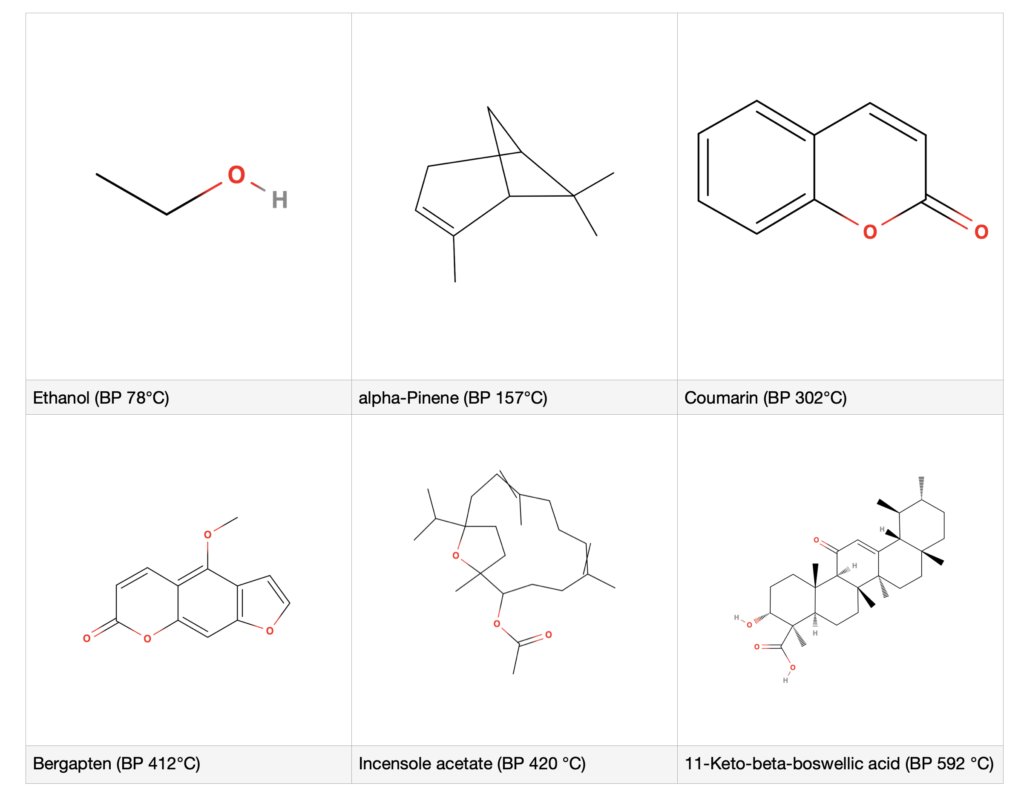
Most EO constituents are VOCs – volatile organic compounds. There are also VVOCs (very volatile organic compounds), SVOCs (semi-volatile organic compounds), and NVOCs (non-volatile organic compounds). This categorization is determined by boiling point (BP) at normal atmospheric pressure:
Table 1: Categories of volatile organic compounds
“Boiling point” is the temperature at which a substance starts to change into gas or vapor, and it varies with atmospheric pressure. Bergapten is non-volatile, since it has a BP of 412 °C (773 °F). Interestingly, the occurrence of semi- and non-volatiles in distilled oils is actually possible. Coumarin, an SVOC, is found in Lavender (Lavandula angustifolia) oil. Furanocoumarins (FCs) like bergapten – which are responsible for the phototoxicity of some expressed citrus oils – can also be found in distilled citrus oils, but the amounts are very much lower than for expressed oils. For example, HPLC analysis showed that a distilled Lemon oil contained 22 times less bergapten (0.00275% and 0.000125%) and 930 times less citropten (0.17943% and 0.000193%) than an expressed Lemon oil, which means that the distilled oil is not phototoxic, even though it contains some FCs (Li et al 2021). In terms of analysis, it’s worth noting that FCs often do show up in GC-MS analysis of expressed citrus oils, but to get a precise quantification, HPLC is very useful.
Several heavier molecules sometimes show up in Frankincense oils at around 1% with GC-MS analysis. These include incensole (BP 428.8 °C) and incensole acetate (BP 420.1 °C) even though technically, these would be classed as NVOCs. However, the BPs of BAs are all in the 500-600 °C range (932-1,112 °F). What this tells us is that the BP classifications are a useful guideline, but they do not always define which molecules will or will not show up in an essential oil. This is because of the conditions of distillation, which can also vary.
Molecular weight is also a consideration. Dr. Rob Pappas, an analytical chemist, also wrote about BAs and Frankincense oils, particularly referring to molecular weight (MW). He observed that the heaviest molecule normally found in distilled oils is sclareol, a diterpene with a MW of 308.5, while boswellic acids are triterpenes, and the MW of 11-keto-β-boswellic acid is 470.7. A general guideline is that molecules with a MW greater than 300 are not volatile, though like BP, this is not an absolute rule.
Dr. Pappas comments: “…our experiments with reference samples of boswellic acid did not detect any trace of said components by GC when attempts were made to analyze dissolved solutions of the pure component, even when the GC run was maxed out at 260 degrees Centigrade and a total run time of 140 minutes (normal GC run times are 30 to 60 minutes). This is very strong evidence that obtaining boswellic acid in an essential oil by steam distillation would be a physical impossibility even at percentages of only a fraction of a percent.”
Solubility is another factor. In a Facebook post, Dr. Pappas points out that he was not able to get even a small amount of boswellic acid to dissolve in Frankincense oil: “…you can blast it with microwaves, or heat it anyway you want to, stir vigorously, hit it with lasers, whatever, that boswellic acid is not going to dissolve in that oil.”
So while it is feasible that BAs are present in Frankincense oils, even if they don’t show up with GC-MS, it is doubtful. Even if present they would likely be in extremely small amounts, especially considering their lack of solubility and their high BPs and MWs. It’s important to appreciate that heavier molecules only pass over during distillation under certain conditions, and the heavier the molecule the less likely it is to pass over at all. Longer distillation times may capture a greater quantity of heavier molecules, but can also lead to a nastier-smelling, and sometimes more toxic, oil.
Reports of boswellic acid in Frankincense oil
Possibly the first time that BAs are mentioned as constituents of Frankincense oil is in a paper by Frank et al (2009), which contains this statement: “One of the main components of frankincense oil is boswellic acid, a component known to have anti-neoplastic properties.” However, the paper contains no evidence that the essential oil contains BAs, only that ethanol and methanol extracts do: “In search of the active medicinal ingredients of frankincense resins, Chevrier et al. reported that ethanol extract of Boswellia carteri resin comprises 7 boswellic acids . Akihisa et al. reported that methanol extract of Boswellia carteri resin consists of 15 triterpene acids, including boswellic acids, and 2 cembrane-type diterpenes .” So the statement that BAs are a “main component” is not supported, and the authors seem to have confused the resin with the essential oil.
This is how myths are born.
The first published analysis of a Frankincense oil containing BAs was by Suhail et al (2011), who reported 30.1 mg/mL (about 3%) of BA in a B. sacra oil distilled at 100 °C (212 °F), and using HPLC analysis. However, in a private communication with Dr. Suhail, an Iraqi chemist living in Oman, he told me that this was achieved using vacuum distillation, and that this fact was not mentioned in the paper. He continued:
“Boswellic acids can be distilled into Boswellia essential oil, under vacuum in well designed equipment. Still it’s not the best nor close to a good way of extracting boswellic acids…To us, presence of boswellic acids in frankincense essential oil means nothing as its nonsignificant.”
Vacuum distillation is sometimes employed when dealing with substances that normally boil at inconveniently high temperatures or that decompose when boiling under atmospheric pressure, as it results in constituents vaporizing at lower temperatures. However, vacuum distillation is not used for commercial production of Frankincense oils.
Let’s look more closely at the reported 3% of BAs. In a subsequent paper, also co-authored by Dr. Suhail (Ni et al 2012) the same data for BAs are reported (so this was done once, not twice), but this paper reveals that the oil was split into four fractions, and the 3% was only found in the heaviest fraction. The lightest fraction only contained 0.09% of BAs, and this from 12 hours of vacuum distillation. (Simply put, fractionation is a process that was used here to divide the essential oil into four different “oils” known as “fractions”, according to the molecular weight of the constituents.)
Dr. Suhail told me: “The paper description of extraction process is wrong . For “nonsense” reasons revisor refused to include our description of extraction process as it is “unusual” and as there has been post-distillation processing (fractionation) preferring to include the basic and common extraction process of frankincense essential oil.” Dr. Suhail sent me this photo of the equipment used, apparently showing that a vacuum unit was included.
Distillation time
Suhail et al also distilled the oil for 12 hours, which is unusually long – most commercial distillation of Frankincense runs for 6-7 hours. The reason for this is that (a) there is no further yield of EO after 7 hours, and (b) if you continue with distillation the oil will not smell good due to reflux and compound degradation in the still. Using a Clevenger apparatus, Yadav et al (2018) found that, using either whole or ground B. serrata resin, essential oil yield did not increase after 7 hours for whole resin, and 6 hours for ground resin.
Trygve Harris, an American B. sacra distiller who lives in Oman, told me: “We usually do about 6-7 hours but we cut off after some time of sesquiterpenes. While we value an active oil, we also value the odor profile and don’t want that oily desperate note one can squeeze out of an extra hour. The BAs stay in the biomass. We use it but distillation is not useful. The oil (in my opinion) smells like crap with a nauseating tail if it’s distilled for too long.” Dr. Suhail also told me that they did find traces of BAs in a Frankincense oil that was distilled for 56 hours, without the need for a vacuum. This would likely produce a relatively unpleasant-smelling oil that would not be commercially viable anyway, because of the distillation time – and you still only get traces of BAs. (A “trace” amount is less than 0.01%.)
A subsequent study reported 0.1% of BAs in distilled B. sacra oil, quantified using HPLC, but there are a few problems with this study (Al-Harrasi et al 2017). No information is given about how the essential oil was extracted – the distillation equipment (in fact there is no reference to distillation at all), the duration or the temperature, and all of these can affect the result, so we do not know whether this can be compared to commercial distillation. One possible reason for this lack of detail is that the main focus of the paper is on methanol extracts, which contained up to 0.6% BAs.
All of this demonstrates that the 3% reported by Suhail et al is not representative, and was only there because of the unusual distillation parameters used, along with fractionation. Even the 0.1% reported by Al-Harrasi et al may not be not reliable due to the lack of detail.
What about Frankincense CO2 extracts?
CO2 extracts of Frankincense oils are also produced, and it is more likely that BA would be found in a CO2 extract than an essential oil, because of the different extraction conditions (solvent, plus pressure). Flavex, a producer of CO2 extracts, states that their B. serrata select CO2 extract contains “traces of boswellic acids”, but for their B. carteri select CO2 extract, no BAs are mentioned. Note that most authorities consider B. sacra and B. carteri to be synonymous, though some claim that they are distinct species.
In conclusion
So far there is no reliable report of BAs in any Frankincense oil produced using normal distillation parameters. BAs do not show up in GC-MS analysis of Frankincense oils, but may be present in trace amounts that will show up with HPLC analysis, and that will likely have no influence on the therapeutic action of the oil. It is entirely possible that some Frankincense oils demonstrate antitumoral properties, whether or not they contain BAs. Although so far there is not much clinical evidence – most of the published studies are in vitro – I am optimistic about the possibilities for skin cancers, and will report on any new developments in this area. BAs are found in substantial amounts in almost any Frankincense extract, and there are several sold online with anything up to 60% BAs.
References
Al-Harrasi, A., Rehman, N. U., Mabood, F., Albroumi, M., Ali, L., Hussain, J., … Alameri, S. (2017). Application of NIRS coupled with PLS regression as a rapid, non-destructive alternative method for quantification of KBA in Boswellia sacra. Spectrochimica Acta – Part A: Molecular and Biomolecular Spectroscopy, 184, 277–285. https://doi.org/10.1016/j.saa.2017.05.018
Frank, M. B., Yang, Q., Osban, J., Azzarello, J. T., Saban, M. R., Saban, R., … Lin, H.-K. (2009). Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complementary & Alternative Medicine, 9, 6. https://doi.org/10.1186/1472-6882-9-6
Li, G., Xiang, S., Pan, Y., Long, X., Cheng, Y., Han, L., & Zhao, X. (2021). Effects of cold-pressing and hydrodistillation on the active non-volatile components in lemon essential oil and the effects of the resulting oils on aging-related oxidative stress in mice. Frontiers in Nutrition, 8(June), 1–15. https://doi.org/10.3389/fnut.2021.689094
Mertens, M., Buettner, A., & Kirchhoff, E. (2009). The volatile constituents of frankincense – a review. Flavour and Fragrance Journal, 24, 279–300. https://doi.org/10.1002/ffj
Ni, X., Suhail, M. M., Yang, Q., Cao, A., Fung, K. M., Postier, R. G., … Lin, H. K. (2012). Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complementary and Alternative Medicine, 12(1), 1. https://doi.org/10.1186/1472-6882-12-253
Niebler, J., & Buettner, A. (2016). Frankincense revisited, part I: Comparative analysis of volatiles in commercially relevant Boswellia species. Chemistry and Biodiversity, 13(5), 613–629. https://doi.org/10.1002/cbdv.201500329
Ojha, P. K., Poudel, D. K., Rokaya, A., Satyal, R., Setzer, W. N., & Satyal, P. (2022). Comparison of volatile constituents present in commercial and lab-distilled frankincense (Boswellia carteri) essential oils for authentication. Plants, 11(16). https://doi.org/10.3390/plants11162134
Roy, N. K., Parama, D., Banik, K., Bordoloi, D., Devi, A. K., Thakur, K. K., … Kunnumakkara, A. B. (2019). An update on pharmacological potential of boswellic acids against chronic diseases. International Journal of Molecular Sciences, 20(17). https://doi.org/10.3390/ijms20174101
Suhail, M. M., Wu, W., Cao, A., Mondalek, F. G., Fung, K.-M., Shih, P.-T., … Lin, H.-K. (2011). Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complementary and Alternative Medicine, 11(1), 1–14. https://doi.org/10.1186/1472-6882-11-129
Van Vuuren, S. F., Kamatou, G. P. P., & Viljoen, A. M. (2010). Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. South African Journal of Botany, 76(4), 686–691. https://doi.org/10.1016/j.sajb.2010.06.001
Yadav, S. (2018). Effect of different parameter on yield of frankincense oil using steam distillation and GC-MS analysis. Journal of Emerging Technologies and Innovative Research, 5(6), 72–77.






Thank you for a wonderfully enlightening article. I’m glad to know that i can look for frankincense extracts if I am interested in the boswellic acids, and glad to be informed that they are not really so present in the essential oil.
Thank you very much for going into the detail of the problems and revealing how the myths are born. I use these types of articles also in the teaching process as examples.
Thank you Robert for this very clear update on the contentious issue of frankincense oil and boswellic acids. It is a most welcome reference piece to help educate those who are being exploited, and misguided, by ‘health advocates’ selling frankincense oil for cancer treatment.
Hello Robert, thank you for your information! I do have a question about Boswellic acids. I purchased a bottle of Boswellia Extract which claims to have 65% Boswellic Acids. My concern is that there is very little fragrance when I open the bottle. Would the Acids be a different product than than actual frankincense and I should not expect to have it smell anything like Frankincense? Perhaps the acids do not have a fragrence?
Hi Brenda, I think it’s fine – boswellic acids are non-volatile so they have very little odor.