Part Two: Skin Healing
by Robert Tisserand
Of the several species of Sandalwood oil commercially produced, this article focuses on just one, Santalum album. Although Santalum album oil (SAO) has been produced in India for centuries, large-scale production there has now entirely ceased and has been replaced by oil from cultivated plantations of Santalum album in Australia. The reasons for this are discussed in detail in the first article of this two-part series. Part one covered four subject areas: history, sustainability challenges, quality control and psychodermatology. This second part focuses on the skin-healing properties of SAO and highlights emerging clinical data, linking it with mechanisms of action.
Skin safety
Patch testing is widely used by dermatologists in order to ascertain what might be causing a patient’s skin condition, and multiple potential allergens are tested at the same time on the upper back. Generally, there is a standard dilution for each test substance, and for SAO this is 2%, but 10% dilutions have also been used. When a substance used in patch testing is linked to the cause of a patient’s skin condition, this is known as clinical relevance. Sometimes patients react to substances that are not clinically relevant.
In terms of patch testing for contact dermatitis (either irritation or allergy), SAO has a good safety profile. Both 10% and undiluted SAO are non-irritant (Burdock and Carabin, 2008). With regard to allergic reactions, in five dermatology reports, 12 of 3,542 patients (0.34%) were sensitive to a 2% dilution of SAO, and in three reports, 69 of 5,595 patients (1.2%) were sensitive to a 10% dilution (Tisserand and Young, 2014). In a subsequent multi-center European study, 656 of 48,956 dermatitis patients (1.38%) showed positive reactions to 10% SAO (Warshaw et al., 2017). In a repeat insult patch test for both skin irritation and sensitization conducted by AMA Laboratories, NY, undiluted SAO produced no adverse reactions in 100 test subjects (Santalis Pharmaceuticals, unpublished data).
A total of four reports of photoallergy testing show that nine of 621 patients (1.45%) tested positive to SAO at 2% (Fotiades et al., 1995; Greenspoon et al., 2013; Scalf et al., 2009; Victor et al., 2009). It should be noted that clinical relevance was generally not established, and photoallergy to essential oils is very rare.
While this is the best information available, the purity of the SAO used in most of these tests is unclear, as there is no transparent traceability of source. Also, it should be noted that dermatitis patients are more prone to allergic skin reactions than healthy subjects, and that statistics from patch testing do not represent real-world risk (Tisserand and Young, 2014). Finally, the reliability of patch testing is questionable, since results can vary significantly depending on which brand of patch is used (Lazarov et al., 2007; Mortz and Andersen, 2010; Sherertz et al., 2001).
Skin healing properties
The therapeutic value of SAO in dermatology can often be ascribed to a combination of antioxidant, anti-inflammatory and antimicrobial properties. In addition, SAO inhibits keratinocyte hyper-proliferation, which is problematic in eczema and psoriasis. Antioxidant action is measured in various ways. SAO scavenged DPPH radicals in vitro only very weakly (Inouye et al., 2010) but significantly enhanced both hepatic glutathione enzymes and superoxide dismutase, when fed to mice (Banerjee et al., 1993; Chhabra and Rao, 1993; Misra and Dey, 2013).
SAO demonstrates significant anti-inflammatory properties. It suppressed the production of 20 of 26 (77%) of tested pro-inflammatory cytokines and chemokines in human dermal fibroblasts (Sharma et al., 2014). Unpublished research by Santalis Pharmaceuticals has confirmed that SAO significantly reduces the production of multiple cytokines and chemokines in reconstituted human skin, in response to inflammation by P. acnes. In reconstituted psoriasis tissue, SAO at 0.002% decreased levels of Interleukin (IL) IL-b, IL-6 and IL-8 by 56%, 75% and 83% respectively (Sharma et al., 2017). Interestingly, SAO reduced inflammation in keratinocytes by inhibiting a pro-inflammatory enzyme, 11b-HSD1 (Itoi-Ochi et al., 2016).
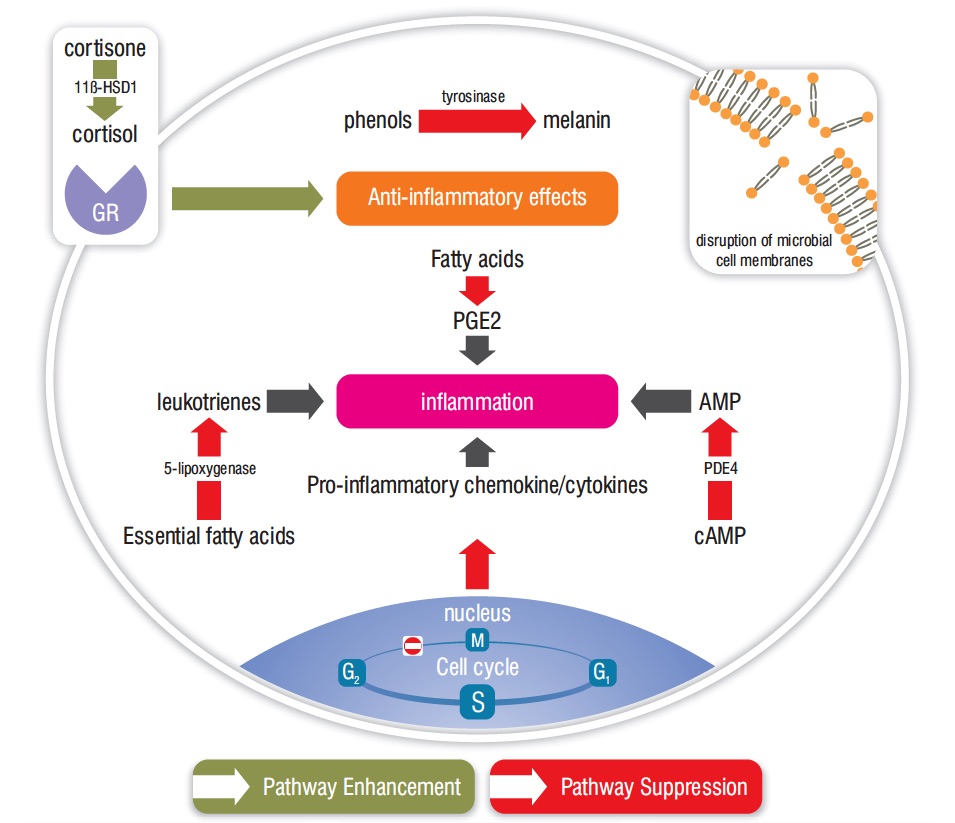
Both α-santalol and β-santalol dose-dependently suppressed prostaglandin E2 production in skin cells, suggesting that the anti-inflammatory action of SAO takes place partly through inhibition of cyclo-oxygenase enzymes (Sharma et al., 2014). In an in vitro study for inhibition of 5-lypoxygenase, SAO was more effective than the essential oils of Blue Cypress (Callitropsis intratropica) and Blue Chamomile (Matricaria recutita) (Baylac and Racine, 2003). Figure 2 illustrates several anti-inflammatory pathways for SAO, and the image at top right shows an important antimicrobial mechanism.
A common side effect of radiation therapy for cancer is skin inflammation and irritation. This is known as radiodermatitis and is related to oxidative stress and an increase in cytokines, including IL-β, IL-6 and IL-8 (De Sanctis et al., 2014). In a nine-week open-label clinical study of 46 head and neck cancer patients undergoing radiotherapy, a proprietary cream (Vicco® Turmeric Skin Cream) containing 16% turmeric extract and 0.5% SAO significantly inhibited the degree of radiodermatitis (24 patients) compared to baby oil (22 patients) (Palatty et al., 2014). In a similar study with the same product in 40 breast cancer patients (20 in each group) radiodermatitis was significantly delayed and mitigated in the sandalwood/turmeric group compared to the baby oil group. Patients had unilateral cancer and had undergone radical mastectomy followed by chemotherapy (Rao et al., 2017). Although this treatment included two active ingredients, it shows a link between antioxidant and anti-inflammatory action, and skin healing/wound healing effects. No adverse events were reported in either study.
Topical infection
SAO has shown activity against a range of bacteria, yeasts and fungi associated with skin disease (Table 1). Perhaps surprisingly, in an in vitro comparison of 24 essential oils, including Lemongrass (Cymbopogon citratus), Clove (Syzygium aromaticum) and Oregano (Origanum vulgare), SAO was the most effective against Candida albicans (Hammer, 1998). SAO dose-dependently inhibited Herpes simplex virus 2 (HSV-2), and was remarkably active against Herpes simplex virus 1 (HSV-1), suggesting a potential use in the treatment of cold sores (Benencia and Courrèges, 1999).
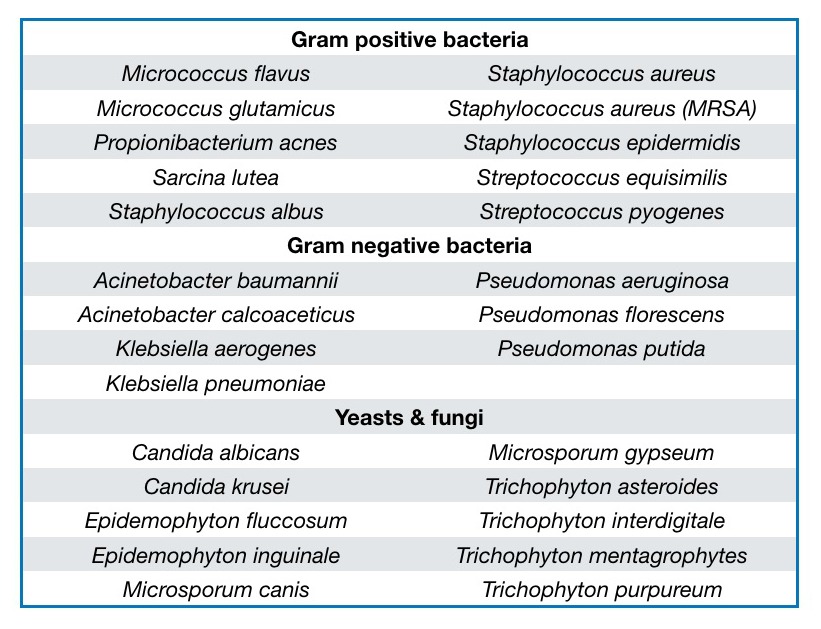
Table 1. Microbes associated with skin disease and against which Santalum album oil has shown activity. After Moy and Levenson, 2017.
Several clinical trials have highlighted the potential clinical use of SAO in infective skin conditions, two of them also including salicylic acid, which is already an FDA-approved active ingredient for these disorders. Acne is a skin condition that occurs when hair follicles become plugged with oil and dead skin cells. Two bacteria in particular tend to proliferate in acne lesions: Staphylococcus epidermidis and Propionibacterium acnes, and both are inhibited in vitro by SAO. Acne is most common among teenagers; it causes whiteheads, blackheads or pimples, and usually appears on the face, forehead, chest, upper back and/or shoulders.
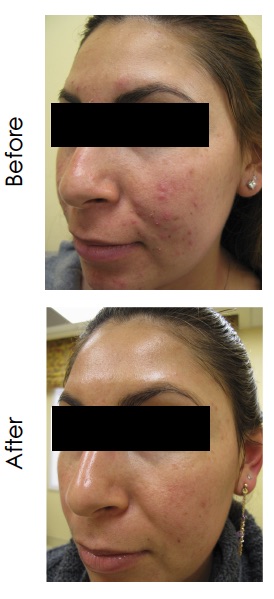
An open-label pilot study of an over-the-counter topical regimen containing 0.5% salicylic acid and up to 2% SAO was conducted in adolescent and adult subjects with mild to moderate facial acne (Figure 3). The regimen consisted of foaming cleanser, serum, spot treatment and mask. Over the course of the eight-week treatment period, 42 of 47 participants (89.4%) experienced an improvement when compared with baseline, using the Global Aesthetic Improvement Scale (GAIS) scale. No adverse events were seen that would limit use of the regimen (Moy et al., 2012).
Common warts (Verruca vulgaris) are small, grainy skin growths that occur most often on fingers or hands. Rough to the touch, common warts often feature a pattern of tiny black dots which are small, clotted blood vessels. Common warts are caused by the human papilloma virus (HPV) and are transmitted by touch. Children and young adults are more likely to develop common warts, as are people with weakened immune systems (Mayo Clinic, 2015).
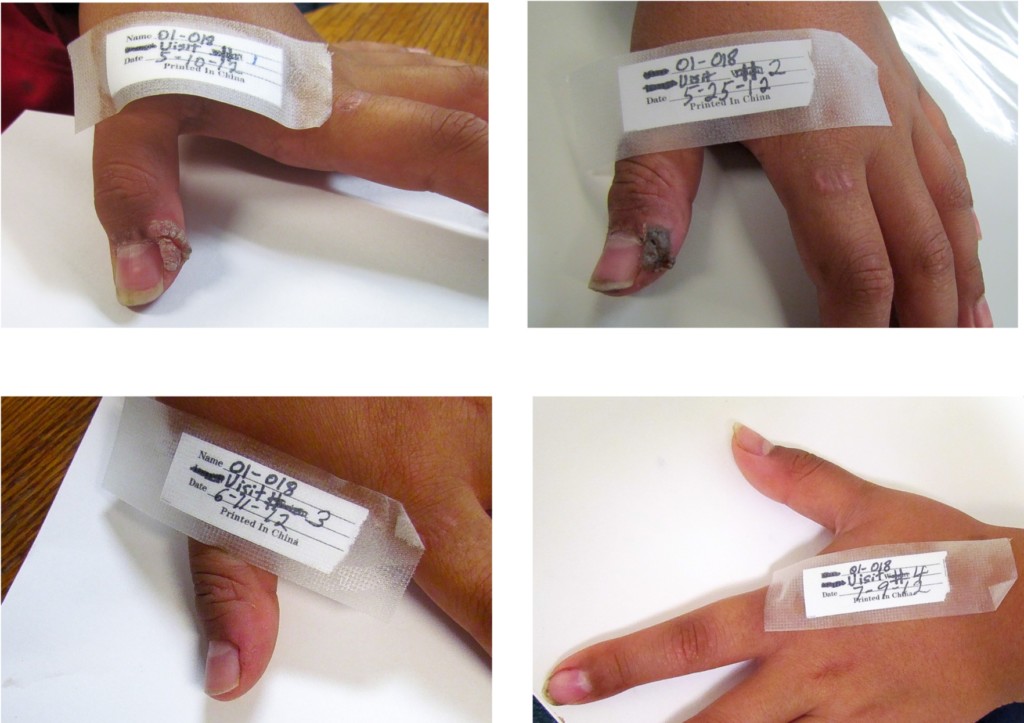
A proprietary topical collodion product containing 17% salicylic acid and approximately 2% α-santalol was used in two open-label pilot studies in children and adolescents with common warts. (Collodion is a yellowish, viscous, highly flammable solution of pyroxylin in ether and alcohol. It is used in medicine chiefly for cementing dressings and sealing wounds.) A total of four of 25 (16%) and seven of 33 (21%) of patients experienced complete resolution of treated warts (Figure 4). The treatment was well tolerated with 10-30% of patients experiencing mild to moderate itching, burning, dryness or stinging, symptoms that are common in wart treatment (Browning et al., 2017a). In a third open-label pilot study, ten subjects, ranging in age from six to adult, applied undiluted SAO to common warts twice daily for 12 weeks. At the end of the study period, 10 of the 12 (80%) had complete resolution of all treated warts, with the other two subjects experiencing moderate improvement. None of the subjects reported skin irritation, redness, pain or other adverse symptoms (Haque and Coury, 2018).
A randomized, double-blind, placebo-controlled dose range finding trial of SAO ointment (10%, 20%, and 30% strengths) was studied in subjects with common warts (Verruca vulgaris) caused by HPV (US National Library of Medicine, 2017a). The primary endpoints of the trial were efficacy, safety, and tolerability. All three treatment dilutions were deemed to be safe and well tolerated. There were no serious adverse events considered to be related to the study medication, and only four adverse events (three in the 30% group and one in the 10% group) were deemed to be related to the study medication; notably, all were mild, reversible irritation at the site of application. All three treatment groups showed greater rates of wart clearance and reduction in wart area than did those in the placebo group (unpublished results, 2016, Santalis Pharmaceuticals).
A clinical trial using SAO for genital warts with 30 participants is currently in progress (US National Library of Medicine, 2017b).
Molluscum contagiosum is a skin condition caused by a poxvirus of the Molluscipox genus, which is transmitted by close physical contact. Preschool and elementary school-aged children are more commonly affected than are other ages. The condition presents as asymptomatic, discrete, smooth, flesh-colored, dome-shaped papules. It typically resolves within months in people without immune deficiency, but treatment may be preferred for social and cosmetic reasons or to avoid spreading the infection (Leung et al., 2017; Van der Wouden et al., 2017).
In a pilot study, nine subjects used a proprietary sandalwood soap (composition unknown) as a treatment for molluscum contagiosum. All subjects experienced complete resolution of the condition within the 12 weeks of the study. No adverse effects were reported (Haque and Coury, 2018). The subjects had contracted the condition an average of 5.3 months before entering the study. This condition may resolve without treatment in as little as six months, but some cases take several years.
Barrier disruption (eczema and psoriasis)
Eczema (atopic dermatitis) is a condition that causes patches of red and itchy skin. It is common in children but can occur at any age. Eczema is long lasting (chronic) and tends to flare periodically. It may be accompanied by asthma or hay fever (Mayo Clinic, 2018a). Psoriasis is a skin condition that causes cells to build up rapidly on the surface of the skin. The extra skin cells form scales and red patches that are itchy and sometimes painful. There is no known cure for psoriasis, but symptoms can be managed (Mayo Clinic, 2018b).
Both eczema and psoriasis are T-cell mediated chronic inflammatory skin diseases characterized by disruption in the integrity of the skin’s barrier function. This is in part associated with depletion of key lipids in the stratum corneum and is associated with keratinocyte hyper-proliferation and faulty keratinocyte differentiation (Sahle et al., 2015). Barrier disruption, in turn, permits a low level of chronic infection, as bacteria that normally populate the skin surface become problematic.
Interim results from an on-going Phase 2 clinical trial, in which 10% SAO was topically applied, show that it is well tolerated and alleviates mild to moderate psoriasis symptoms. An anhydrous excipient was used, primarily consisting of caprylic/capric triglyceride (Sharma et al., 2017). The researchers hypothesized that SAO might provide therapeutic benefit to psoriasis patients due to its anti-inflammatory and anti-proliferative properties, seen in skin cells (Dickinson et al., 2014; Itoi-Ochi et al., 2016; Sharma et al., 2014). Also, a clinical anti-inflammatory benefit had previously been shown in the Phase 2 study of acne patients already cited (Moy et al., 2012).
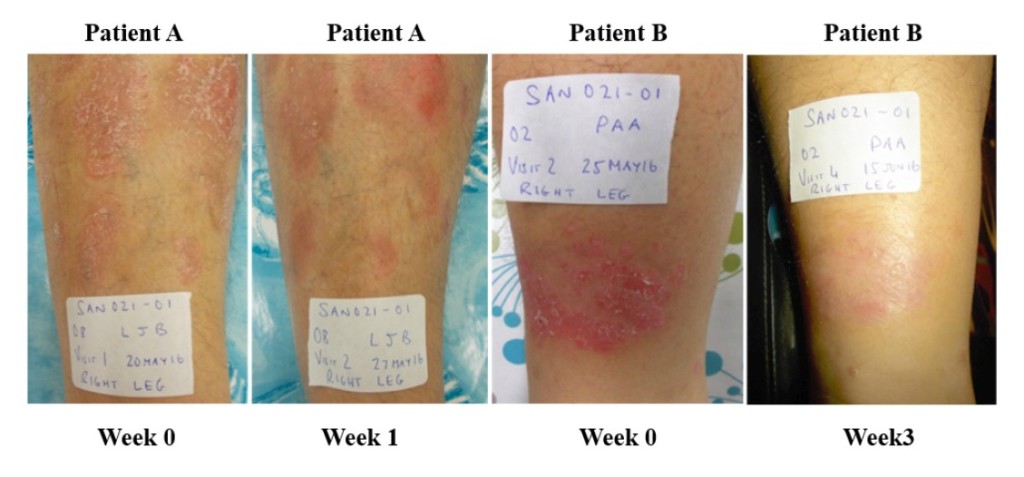
Figure 5. Psoriasis lesions in two patients – before treatment, and after 1 week (patient A) and 3 weeks (patient B). Creative Commons Attribution License, Sharma et al., 2017.
In nine of the 11 subjects in the psoriasis trial who could be evaluated, the severity of psoriatic plaques was reduced by the end of the study. One patient withdrew from the study with a mild adverse event after three weeks, and their skin reaction resolved. The average immunoglobulin A (IGA) score was significantly reduced by one week and continued to improve at two and four weeks. Overall, 64% of the subjects (7/11) demonstrated a 1.0 or greater reduction in their IGA score during the 28-day treatment period. Two examples are shown of lesions before and after either one or three weeks of treatment (Figure 5). These were both scored as markedly improved. Two additional patients demonstrated moderate improvement. These clinical observations demonstrate that SAO can provide symptom relief for psoriasis patients.
Further research evaluated the ability of SAO to affect the psoriatic phenotype using psoriatic and normal human skin models. SAO had no impact on the phenotype of the normal skin tissue model; however, SAO treatment of the psoriasis tissue model reversed psoriatic pathology. This supports the hypothesis that the clinically observed symptom alleviation is due to suppression of intrinsic tissue inflammation in afflicted lesions (Sharma et al., 2017).
It has been suggested that Helicobacter pylori infection in the stomach might be a triggering factor in psoriasis, probably via inflammatory pathways. H. pylori infections are considerably more common in psoriasis patients than in healthy controls, and there are several reports of cases in which psoriatic lesions cleared up following the eradication of H. pylori infections (Magen and Delgado, 2014). This is noteworthy, since SAO constituents, including α-santalol and β-santalol, are strongly active against a clarithromycin-resistant strain (TS281) as well as other strains of H. pylori (Ochi et al., 2005).
Psoriasis patients suffer from impaired quality of life, psychosocial problems and emotional distress, and stress and infection are two of the triggers that can initiate the inflammatory process resulting in keratinocyte hyper-proliferation (Sharma et al., 2017). Since stress, barrier disruption, infection and inflammation all cross-promote each other, remedies that address all four factors are arguably most likely to succeed. The anti-stress effects of SAO, and the related study of psychodermatology, are discussed in the first article of this two-part series.
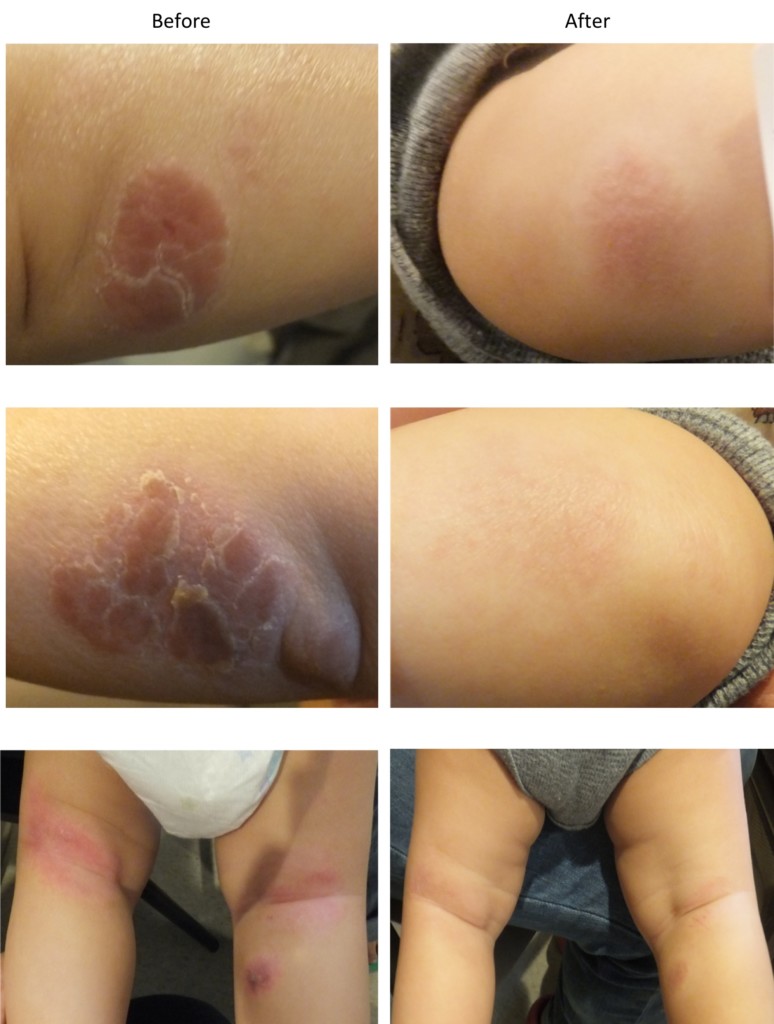
Figure 6. Pediatric eczema in one patient, before treatment and after 60 days. Browning et al., 2017b.
Eczema also appears to be amenable to treatment with SAO. In an open-label study over 60 days, 22 children with eczema (aged three months to 11 years) were treated with three products (daily cleanser, soothing cream and bubble bath gel) all containing 0.1% colloidal oatmeal and SAO. After one week, 91% showed improvement in Eczema Area and Severity Index (EASI) scores. At the end of the trial, 18 children had a 25% or greater reduction in EASI score, and nine were completely clear of eczema or had a greater than 90% EASI score improvement (Figure 6). Adverse events were mild to moderate, and none were considered to be related to the treatment regimen (Browning et al., 2017b). Two further clinical trials are currently in progress, each in 72 eczema patients, using a 5% preparation of SAO, and encompassing a wide age range (US National Library of Medicine, 2017c; US National Library of Medicine, 2017d). Recent interest in inflammation-specific targets for the treatment of skin conditions such as psoriasis and eczema has focused on substances that reduce levels of IL-17 and the activity of PDE412 (Moy and Levenson, 2017). SAO has been shown to specifically inhibit both targets in various in vitro models (Sharma et al., 2017; Sharma et al., unpublished material). This suggests likely mechanisms for the therapeutic activity seen in the clinical studies of SAO in the treatment of these skin conditions.

Figure 7. Santalum album, showing lighter colored sapwood and darker colored heartwood © Quintis Limited.
Skin cancer
Skin cancers are due to the development of abnormal cells that may spread to other parts of the body (National Cancer Institute, 2015). There are three main types: basal-cell skin cancer (BCC), squamous-cell skin cancer (SCC) and melanoma (National Cancer Institute, 2018a). The first two, along with some less common skin cancers, are known as non-melanoma skin cancer (NMSC). Basal-cell cancer grows slowly and can damage the tissue around it but is unlikely to spread to distant areas or result in death. Squamous-cell skin cancer is more likely to spread (Cakir et al., 2012). It usually presents as a hard lump with a scaly top but may also form an ulcer (Dunphy, 2011). Melanomas are the most aggressive. Signs include a mole that has changed in size, shape, or color, has irregular edges, has more than one color, is itchy or bleeds (National Cancer Institute, 2018b). More than 90% of skin cancer cases are caused by exposure to ultraviolet radiation from the sun. This exposure increases the risk of all three main types of skin cancer (Gallagher et al., 2010).
In terms of the number of papers published, the most widely researched use of SAO oil in dermatology is for skin cancer, though to date there are no human studies. α-Santalol was significantly effective when tested against human epidermoid carcinoma or melanoma cells in vitro (Kaur et al., 2005; Zhang et al., 2010) and topical application showed good efficacy inhibiting UVB-induced skin cancer in mice when used at 10% (Santha and Dwivedi, 2013) or 5% (Arasada and Bommareddy, 2008; Bommareddy et al., 2007; Chilampalli et al., 2013; Dwivedi et al., 2006). The 5% protocol was also effective for both α-santalol and β-santalol for chemical-induced skin cancer in mice (Dwivedi et al., 2003; Kim et al., 2006).
The antitumoral mechanisms of action for α-santalol are summarized by Zhang and Dwivedi (2011) and include apoptosis and inhibition of cell growth at the G2/M phase. Dickinson et al. (2014) observed that low concentrations of SAO inhibited rapid proliferation of keratinocytes and suggest that the oil may reduce the risk of actinic keratosis and skin cancer. An Australian practitioner found that a mix of essential oils including 13% Santalum spicatum was an effective treatment in several cases of actinic keratosis (Tisserand Institute, 2011). An actinic keratosis, also known as a solar keratosis, is a rough, scaly patch on the skin that develops from years of exposure to the sun. It is most commonly found on face, lips, ears, backs of hands, forearms, scalp or neck (Mayo Clinic, 2018c).
Applied at 5% topically, SAO inhibited chemical-induced skin cancer in mice (Dwivedi and Abu-Ghazaleh, 1997) and the 5% dilution was more effective than 1.25%, 2.5% or 3.75% (Dwivedi and Zhang, 1999). Although there has been no clinical research, SAO shows promise in terms of both prevention and treatment for skin cancers. For treatment, 5-10% concentration of SAO might be appropriate. For prevention, lower concentrations would make sense as the conditions of mouse testing were quite severe.
For other cancers, SAO has shown promise in in vitro testing with human cells for bladder, colon, and breast cancers, α-santalol for liver cancer, breast cancer (both ER-positive and ER-negative) and prostate cancer, β-santalol for leukemia, and both α-santalol and β-santalol for oral cancer (Bommareddy et al., 2012, 2015; Dozmorov et al., 2014; Lee et al., 2015; Matsuo et al., 2014; Mitoshi et al., 2012; Ortiz et al., 2016; Santha et al., 2013; Saraswati et al., 2013a, 2013b). Topically applied at 10% to female rats, α-santalol demonstrated good transdermal absorption, and in breast cancer it significantly reduced tumor incidence (Dave et al., 2017).
Whether SAO would be an effective anticancer treatment is not known, but the above findings suggest its use in prevention.
Summary
Santalum album oil (SAO) is proving to be an effective treatment for a wide range of skin conditions. Data from patch testing suggest a low level of risk, and this is borne out by clinical results, which show encouraging risk-benefit ratios. In vitro, and some in vivo studies, demonstrate properties that include antibacterial, antifungal, antiviral, anti-inflammatory and antitumoral effects, and some mechanisms of action have been elucidated. The efficacy of SAO in terms of anti-inflammatory and antimicrobial action compared with certain other essential oils may be surprising. Clinical studies are ongoing, and show promise in the treatment of facial acne, common warts, psoriasis, radiodermatitis, molluscum contagiosum and eczema. Concentrations varying from 2% to 10% (up to 100% for common warts) have been used in a variety of excipients. Other skin conditions that may be amenable to SAO therapy include cold sores, sensitive skin, rosacea, genital warts, skin cancers, and fungal infections such as onychomycosis, diaper rash and athlete’s foot.
Acknowledgements
Thanks to Dr. Corey Levenson of Santalis Pharmaceuticals (Antonio, TX) for assistance in providing images and unpublished material, and to Quintis Limited (Australia) for supplying images.
Notes
The suppliers given for SAO in the reports cited include Cauvery (Bangalore), Dragoco (Austria), Karnataka Emporium (New Delhi), Mountain Rose Herbs (OR), NOW Foods (IL), Organic Infusions (CA), Phytoaroma Labs (Yokohama), Santalis Pharmaceuticals (TX), Shiseido (Japan), Synthite Industrial Chemicals (Cochin), and Young Living (UT). Haque and Coury (2018) gave no source. Only two papers (Misra and Dey, 2013 and Sharma et al., 2017) included a detailed analysis of the essential oil used.
This article was also published in the International Journal of Professional Holistic Aromatherapy Vol. 7, Issue 1 (Summer 2018).
References
Arasada B and Bommareddy A. (2008). Effects of santalol on proapoptotic caspases and p53 expression in UVB irradiated mouse skin. Anticancer Research. 28, p129–132.
Banerjee S, Ecavade A, Rao R. (1993). Modulatory influence of sandalwood oil on mouse hepatic glutathione S-transferase activity and acid soluble sulphydryl level. Cancer Letters. 68 (2–3), p105–109.
Baylac S and Racine P. (2003). Inhibition of 5-lipoxygenase by essential oils and other natural fragment extracts. International Journal of Aromatherapy. 13 (2–3), p138–142.
Benencia F and Courrèges M C. (1999). Antiviral activity of sandalwood oil against herpes simplex viruses-1 and -2. Phytomedicine. 6 (2), p119–123.
Bommareddy A, Crisamore K, Fillman S, et al. (2015). Survivin down-regulation by α-santalol is not mediated through PI3K-AKT pathway in human breast cancer cells. Anticancer Research. 35, p5353–5357.
Bommareddy A, Hora J, Cornish B, et al. (2007). Chemoprevention by α-santalol on UVB radiation-induced skin tumor development in mice. Anticancer Research. 27, p2185–2188.
Bommareddy A, Rule B, VanWert A. (2012). α-Santalol, a derivative of sandalwood oil, induces apoptosis in human prostate cancer cells by causing caspase-3 activation. Phytomedicine. 19, p804–811.
Browning J C, Rock J, Levenson C, et al. (2017a). Open-label marketing trials to evaluate an over-the-counter (OTC) 17% salicylic acid regimen containing highly purified Sandalwood oil for the treatment of common warts (Verruca vulgaris) in pediatrics. Presented as a poster at the Orlando Dermatology & Clinical Aesthetic Conference, Doral, FL.
Browning J C, Rock J, Levenson C, et al. (2017b). Safety, tolerability and efficacy of a novel regimen containing 0.1% colloidal oatmeal and East Indian Sandalwood oil (EISO) for the treatment of mild, moderate and severe pediatric eczema (atopic dermatitis) – results of a single-center, open-label study. Presented as a poster at the Orlando Dermatology & Clinical Aesthetic Conference, Doral, FL.
Burdock G A and Carabin I G. (2008). Safety assessment of sandalwood oil (Santalum album L.). Food and Chemical Toxicology. 46, p421–432.
Cakir B Ö, Adamson P, Cingi C. (2012). Epidemiology and economic burden of nonmelanoma skin cancer. Facial plastic surgery clinics of North America. 20 (4), p419–422.
Chhabra S K and Rao A R. (1993). Postnatal modulation of xenobiotic metabolizing enzymes in liver of mouse pups following translactational exposure to sandalwood oil. Nutrition Research. 13 (10), p1191–1202.
Chilampalli C, Zhang X, Kaushik R S, et al. (2013). Chemopreventive effects of combination of honokiol and magnolol with α-santalol on skin cancer developments. Drug Discoveries & Therapeutics. 7 (3), p109–115.
Dave K, Alsharif F M, Islam S, et al. (2017). Chemoprevention of breast cancer by transdermal delivery of α-santalol through breast skin and mammary papilla (nipple). Pharmaceutical Research. 34 (9), p1897–1907.
De Sanctis V, Agolli L, Visco V, et al. (2014). Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy. BioMed Research International. 2014, p1–7.
Dickinson S E, Olson E R, Levenson C, et al. (2014). A novel chemopreventive mechanism for a traditional medicine: East Indian sandalwood oil induces autophagy and cell death in proliferating keratinocytes. Archives of Biochemistry and Biophysics. 558, p143–152.
Dozmorov M, Yang Q, Wu W, et al. (2014). Differential effects of selective frankincense (Ru Xiang) essential oil versus non-selective sandalwood (Tan Xiang) essential oil on cultured bladder cancer cells: a microarray and bioinformatics study. Chinese Medicine. 9 (18), p1–12.
Dunphy L M. (2011). Primary Care: The Art and Science of Advanced Practice Nursing. Philadelphia, PA: F.A. Davis. p242.
Dwivedi C and Abu-Ghazaleh A. (1997). Chemopreventive effects of sandalwood oil on skin papillomas in mice. European Journal of Cancer Prevention. 6, p399–401.
Dwivedi C and Zhang Y. (1999). Sandalwood oil prevents skin tumour development in CD1 mice. European Journal of Cancer Prevention. 8, p449–455.
Dwivedi C, Guan X, Harmsen W. (2003). Chemopreventive effects of α-santalol on skin tumor development in CD-1 and SENCAR mice. Cancer Epidemiology, Biomarkers & Prevention. 12, p151–156.
Dwivedi C, Valluri H B, Guan X, et al. (2006). Chemopreventive effects of α-santalol on ultraviolet B radiation-induced skin tumor development in SKH-1 hairless mice. Carcinogenesis. 27 (9), p1917–1922.
Fotiades J, Soter N A, Lim H W. (1995). Results of evaluation of 203 patients for photosensitivity in a 7.3-year period. Journal of the American Academy of Dermatology. 33 (4), p597–602.
Gallagher R, Lee T K, Bajdik C D, Borugian M. (2010). Ultraviolet radiation. Chronic diseases in Canada. 29 Suppl 1, p51–68.
Greenspoon J, Ahluwalia R, Juma N, Rosen C F. (2013). Allergic and photoallergic contact dermatitis: A 10-year experience. Dermatitis. 24 (1), p29–32.
Hammer K. (1998). In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. Journal of Antimicrobial Chemotherapy. 42, p591–595.
Haque M and Coury D L. (2018). Topical sandalwood oil for common warts. Clinical Pediatrics. 57 (1), p93–95.
Haque M and Coury D L. (2017). Treatment of molluscum contagiosum with an East Indian sandalwood oil product. Journal of Dermatological Treatment. In Press.
Inouye S, Takahashi M, Abe S. (2010). Composition, antifungal and radical scavenging activities of 15 rare essential oils. Japanese Journal of Medical Mycology. 4, p1–10.
Itoi-Ochi S, Matsumura S, Terao M, et al. (2016). Sandalwood oil downregulates skin inflammation through 11β-HSD1 activation in keratinocytes. Journal of Dermatological Science. 84 (1), e134.
Kaur M, Agarwal C, Singh R P et al. (2005). Skin cancer chemopreventive agent, α-santalol, induces apoptotic death of human epidermoid carcinoma A431 cells via caspase activation together with dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 26 (2), p369–380.
Kim T H, Ito H, Hatano T, et al. (2006). New antitumor sesquiterpenoids from Santalum album of Indian origin. Tetrahedron. 62 (29), p6981–6989.
Lazarov A, David M, Abraham D, Trattner A. (2007). Comparison of reactivity to allergens using the TRUE Test and IQ chamber system. Contact Dermatitis. 56 (3), p140–145.
Lee B, Bohmann J, Reeves T, et al. (2015). α- and β-santalols directly interact with tubulin and cause mitotic arrest and cytotoxicity in oral cancer cells. Journal of Natural Products. 78 (6), p1357–1362.
Leung, A K C, Barankin B, Hon K L E. (2017). Molluscum contagiosum: An update. Recent Patents on Inflammation & Allergy Drug Discovery. 11 (1), p22–31.
Magen E and Delgado J S. (2014). Helicobacter pylori and skin autoimmune diseases. World Journal of Gastroenterology. 20 (6), p1510–1516.
Matsuo Y, Sakagami H, Mimaki Y. (2014). A rare type of sesquiterpene and β-santalol derivatives from Santalum album and their cytotoxic activities. Chemical & Pharmaceutical Bulletin. 62 (12), p1192–1199.
Mayo Clinic. (2015). Common warts. Available: https://www.mayoclinic.org/diseases-conditions/common-warts/symptoms-causes/syc-20371125. Last accessed 5 May 2018.
Mayo Clinic. (2018a). Atopic dermatitis (eczema). Available: https://www.mayoclinic.org/diseases-conditions/atopic-dermatitis-eczema/symptoms-causes/syc-20353273. Last accessed 5 May 2018.
Mayo Clinic. (2018b). Psoriasis. Available: https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840. Last accessed 5 May 2018.
Mayo Clinic. (2018c). Actinic keratosis. Available: https://www.mayoclinic.org/diseases-conditions/actinic-keratosis/symptoms-causes/syc-20354969. Last accessed 5 May 2018.
Misra B B and Dey S. (2013). Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine. 20 (5), p409–416.
Mitoshi M, Kuriyama I, Nakayama H, et al. (2012). Effects of essential oils from herbal plants and citrus fruits on DNA polymerase inhibitory, cancer cell growth inhibitory, antiallergic, and antioxidant activities. Journal of Agricultural and Food Chemistry. 60, p11343–11350.
Mortz C G and Andersen K E. (2010). Fragrance mix I patch test reactions in 5006 consecutive dermatitis patients tested simultaneously with TRUE Test® and Trolab® test material. Contact Dermatitis. 63 (5), p248–253.
Moy R L, Levenson C, So J J, et al. (2012). Single-center, open-label study of a proprietary topical 0.5% salicylic acid-based treatment regimen containing sandalwood oil in adolescents and adults with mild to moderate acne. Journal of Drugs in Dermatology. 11 (12), p1403–1408.
Moy R L and Levenson C. (2017). Sandalwood album oil as a botanical therapeutic in dermatology. Journal of Clinical and Aesthetic Dermatology. 10 (10), p34–39.
National Cancer Institute. (2015). What is cancer? Available: https://www.cancer.gov/about-cancer/understanding/what-is-cancer. Last accessed 5 May 2018.
National Cancer Institute. (2018a). Skin Cancer Treatment (PDQ®)–Health Professional Version. Available: https://www.cancer.gov/types/skin/hp/skin-treatment-pdq#section/all. Last accessed 5 May 2018.
National Cancer Institute. (2018b). Skin Cancer Treatment (PDQ®)–Health Patient Version. Available: https://www.cancer.gov/types/skin/patient/melanoma-treatment-pdq#section/all. Last accessed 5 May 2018.
Ochi T, Shibata H, Higuti T, et al. (2005). Anti-helicobacter pylori compounds from Santalum album. Journal of Natural Products. 68 (6), p819–824.
Ortiz C, Morales L, Sastre M, et al. (2016). Cytotoxicity and genotoxicity assessment of sandalwood essential oil in human breast cell lines MCF-7 and MCF-10A. Evidence-Based Complementary and Alternative Medicine. 2016, p1–13.
Palatty P L, Azmidah A, Rao S, et al. (2014). Topical application of a sandalwood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. The British Journal of Radiology. 87 (1038), p1–10.
Rao S, Hegde S, Baliga-Rao M, et al. (2017). Sandalwood oil and turmeric-based cream prevents ionizing radiation-induced dermatitis in breast cancer patients: Clinical study. Medicines. 4 (3), p1–8.
Sahle F F, Gebre-Mariam T, Dobner B, et al. (2015). Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacology and Physiology. 28 (1), p42–55.
Santha S, Bommareddy A, Rule B, et al. (2013). Antineoplastic effects of α-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis. PloS One. 8 (2), p1–12.
Santha S and Dwivedi C. (2013). α-Santalol, a skin cancer chemopreventive agent with potential to target various pathways involved in photocarcinogenesis. Photochemistry and Photobiology. 89 (4), p919–926.
Saraswati S, Kumar S, Alhaider A. (2013a). α-Santalol inhibits the angiogenesis and growth of human prostate tumor growth by targeting vascular endothelial growth factor receptor 2-mediated AKT/mTOR/P70S6K signaling pathway. Molecular Cancer. 12 (147), p1–18.
Saraswati S, Kanaujia P K, Agrawal S S. (2013b). α-Santalol demonstrates antitumor and antiangiogenic activities in models of hepatocellular carcinoma in vitro and in vivo. Digestive & Liver Disease. 45 (Suppl. 3), S249-S250.
Scalf L A, Davis M D P, Rohlinger A L, Connolly S M. (2009). Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 20 (1), p44–52.
Sharma M, Levenson C, Bell R H, et al. (2014). Suppression of lipopolysaccharide-stimulated cytokine / chemokine production in skin cells by sandalwood oils and purified α-santalol and β-santalol. Phytotherapy Research. 28 (6), p925–932.
Sharma M, Levenson C, Clements I, et al. (2017). East Indian Sandalwood Oil (EISO) alleviates inflammatory and proliferative pathologies of psoriasis. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2017.00125
Sharma M, Levenson C, Clements I, et al. Antiinflammatory and anti-proliferative effects of East Indian sandalwood oil (EISO): mitigation of the effects of IL-17 on a psoriasis tissue model. Manuscript in preparation.
Sharma M, Levenson C, Clements I, et al. (2018). East Indian sandalwood oil (EISO) is an inhibitor of phosphodiesterase 4 (PDE 4): a new therapeutic option in the treatment of inflammatory skin disease. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2018.00200
Sherertz E F, Fransway A F, Belsito D V et al. (2001). Patch testing discordance alert: False-negative findings with rubber additives and fragrances. Journal of the American Academy of Dermatology. 45 (2), p313–314.
Tisserand Institute. (2011). Ron Guba on essential oils and cancer. Available: http://roberttisserand.com/2011/07/ron-guba-on-essential-oils-and-skin-cancer/. Last accessed 5 May 2018.
Tisserand R and Young R. (2014). Essential oil safety: a guide for health care professionals, 2nd ed. Churchill Livingstone, London. p75, 418.
US National Library of Medicine. (2017a). A Trial of a Botanical Drug Containing East Indian Sandalwood Oil (EISO) for Treatment of Common Warts. Available: https://clinicaltrials.gov/ct2/show/NCT01286441. Last accessed 5 May 2018.
US National Library of Medicine. (2017b). A Trial of a Botanical Drug Containing East Indian Sandalwood Oil (EISO) for the Treatment of External Genital Warts. Available: https://clinicaltrials.gov/ct2/show/NCT03158974?term=sandalwood+oil&rank=8. Last accessed 5 May 2018.
US National Library of Medicine. (2017c). A Trial of a Botanical Drug Product Containing East Indian Sandalwood Oil (EISO) For Treatment of Atopic Dermatitis. Available: https://clinicaltrials.gov/ct2/show/NCT03000595. Last accessed 5 May 2018.
US National Library of Medicine. (2017d). A Trial of a Botanical Drug Containing East Indian Sandalwood Oil (EISO) For Treatment of Atopic Dermatitis. Available: https://clinicaltrials.gov/ct2/show/NCT02871479. Last accessed 5 May 2018.
Van Der Wouden J C, Van Der Sande, R, Kruithof E J, et al. (2017). Interventions for cutaneous molluscum contagiosum. Evidence-based Child Health: A Cochrane Review Journal. 6 (5), p1550–1599.
Victor F C, Cohen D E, Soter N A. (2010). A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. Journal of the American Academy of Dermatology. 62 (4), p605–610.
Warshaw E M, Zug K A, Belsito D V et al. (2017). Positive patch-test reactions to essential oils in consecutive patients from North America and Central Europe. Dermatitis. 28 (4), p246–252.
Zhang X and Dwivedi C. (2011). Skin cancer chemoprevention by α-santalol. Frontiers in Bioscience. S3, p777–787.
Zhang X, Chen W, Guillermo R et al. (2010). α-Santalol, a chemopreventive agent against skin cancer, causes G2/M cell cycle arrest in both p53-mutated human epidermoid carcinoma A431 cells and p53 wild-type human melanoma UACC-62 cells. BMC Research Notes. 3, p1–15.





Hi Rob, appreciate the scientific evidence you use to back up the effectiveness of the oils. i just bought your sandalwood oil 2 ml bottle from amazon. it states that it is the australian version for sustainability, is this still effective for clearing hpv? thanks!
Thank you for your kind comments – though just to be clear I no longer work with Tisserand Aromatherapy. Yes, if it’s from Santalum album, it will be as effective as any Sandalwood oil.
Hello,
Iam unable to locate part one of this article. Could you help please?
Santalum Album Oil Rejuvenated – Part Two: Skin Healing
Thanks in advance,
Tammy
Greetings, You can find Part 1 at the following link: https://tisserandinstitute.org/santalum-album-oil-rejuvenated/
~Shane