by Ginger L. Moore
The online aromatherapy community has been overflowing with DIY recipes for anything from facial creams to home sanitizing sprays.
The push to “do it yourself” is seen everywhere, personal care products included. Those who extol the virtues of the homemade and DIY approach claim the following:
• “It saves you money.”
• “It’s safer than using commercial retail products. ‘Natural’ is better, and then you know exactly what’s in it!”
• “So easy that anyone can do it! No expensive education or training required!”
While all of this is true, to some extent, this approach sometimes results in people overestimating their ability to properly and safely formulate DIY products for the use at home. Just like cooking, some recipes are easy and fool-proof, while others need a bit of skill and extra knowledge (and some really cannot be done unless you have a cosmetic chemist on the line, but that is a story for another day).
When making your own products, safe formulation can be the difference between seeing beneficial results and suffering adverse effects. And water, which is the breath of life, can be an unexpected enemy in our products. Water and water-based mediums (such as aloe, witch hazel, hydrosols, etc.) offers a prime growing habitat for microbes, which are on and in everything in our environment. While some microbes are beneficial, like normal gut bacteria (which help us to digest our food),
others can have negative effects.
Many ‘natural’ ingredients (aloe, plant extracts or infusions, etc.) used in home-prepared products provide ‘food’ for these microbes, contributing to their growth. Hence, water-based products like body or room/linen sprays, cleaning sprays, lotions, creams or gels must include a preservative system to inhibit microbial contamination and growth. The last thing you’d want in your sanitation spray is for the preparation itself be full of the very same germs you wanted to get rid of in the first place.
“Many recipes you’ll come across for DIY aromatherapy sprays are merely water with essential oils or possibly another or additional water component such as witch hazel or vinegar with instructions to shake before using. Unfortunately, these lack proper solubilization and dilution in addition to being a microbe’s playground. Just as often, some form of alcohol is recommended in aromatherapy spray formulations.”
Professional aromatherapists know their subject well and are worth their weight in gold. But they’re often not trained in or lack the knowledge of product formulation, especially hydrous (made with water) products. Many recipes you’ll come across for DIY aromatherapy sprays are merely water with essential oils or possibly another or additional water component such as witch hazel or vinegar with instructions to shake before using. Unfortunately, these lack proper solubilization and dilution in addition to being a microbe’s playground. Just as often, some form of alcohol is recommended in aromatherapy spray formulations. But is that safe?
Essential oil safety is a huge part of what the Tisserand Institute is about, and since many in the aromatherapy community regularly make personal and home care products with them, I’ve been asked to talk about how drinkable grain alcohol (ethanol) can be simply and safely used at home in products such as body or room sprays to:
• Eliminate microbial contamination (act as a preservative)
• Properly dissolve the essential oils for safe use
I will address these and a few basics of formulation with water bases in a form of a Q&A, guiding you through the creation of a water-based spray, one of the most commonly made DIY products. Let’s get started.
Q: What is a preservative?
A: A preservative is defined as a chemical substance that helps slow or prevent the growth of living microorganisms in a wide range of products including foods, medicines and body care products. Living microorganisms that commonly contaminate products are bacteria, mold and fungi. Examples of simple preservatives used in food preservation are salt and vinegar. High proof alcohol is another that can be used in a variety of consumer products, at appropriate and proper concentrations.
Q: What exactly is ethanol (grain) alcohol and can it be used as a preservative?
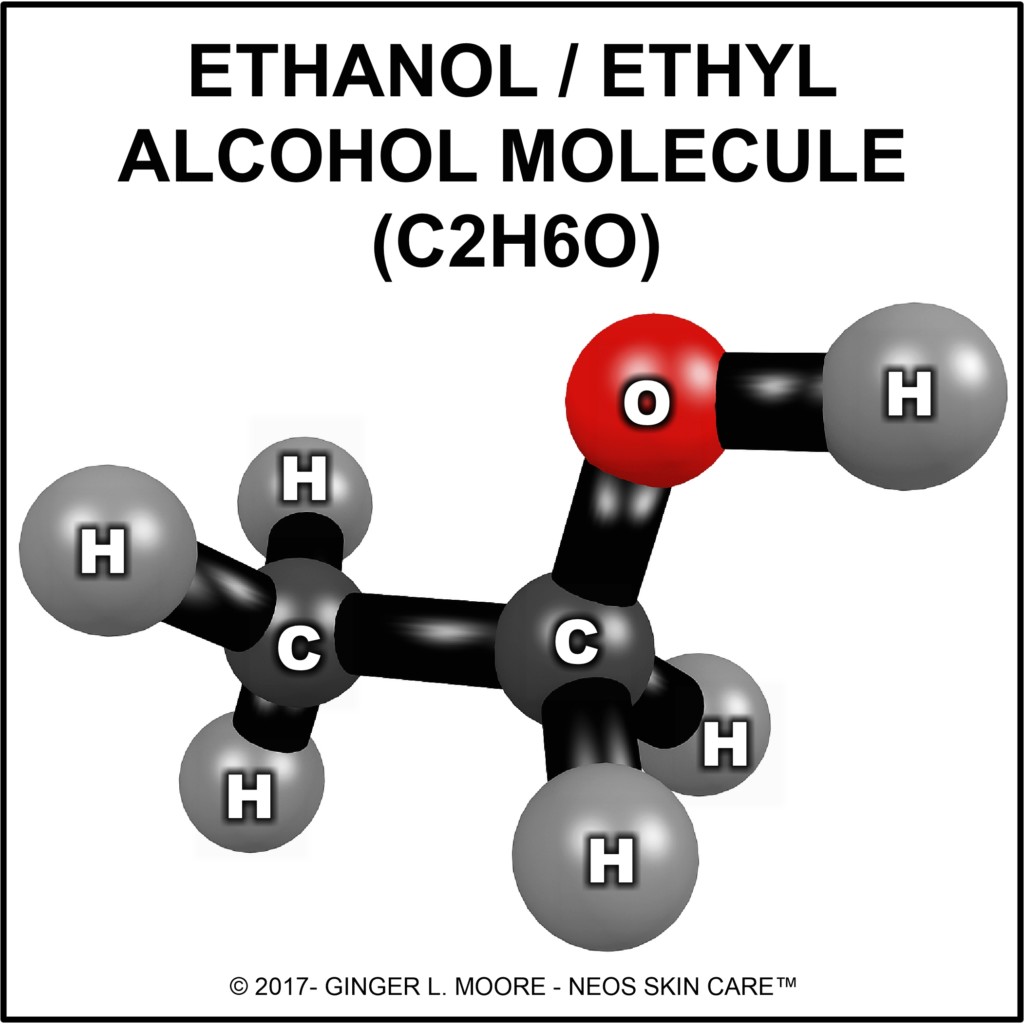
A: Ethanol/ethyl alcohol (C2H6O) is a simple chemical compound. Its molecules are made up of two carbon (C) atoms, six hydrogen (H) atoms, and one oxygen (O) atom. A water soluble, volatile and flammable solution, ethanol (ETOH) is obtained through fermentation and distillation of starchy plant matter such as grains, beets, fruits, and sugars.
While ethanol may be a simple chemical compound, it has many uses. It’s found in a variety of goods including household and industrial cleaners, perfumes and cosmetics, medicines, foods and of course alcoholic beverages.
Ethanol is “cidal” in nature, meaning it acts to kill many living microorganisms. Because it has the ability to destroy microbes, it has been used in medicine and for sanitation purposes for hundreds of years. Since it also carries preservative qualities by inhibiting microbial growth in some solutions and mixtures, ethanol can also be used accordingly for this purpose in a variety of products, at the proper concentration and amounts.
Q: How does ethanol kill microbes?
A: Looking at the anatomy of a bacterial cell and the basic structure of an alcohol molecule, we can get a visual understanding of how alcohol destroys and kills bacteria.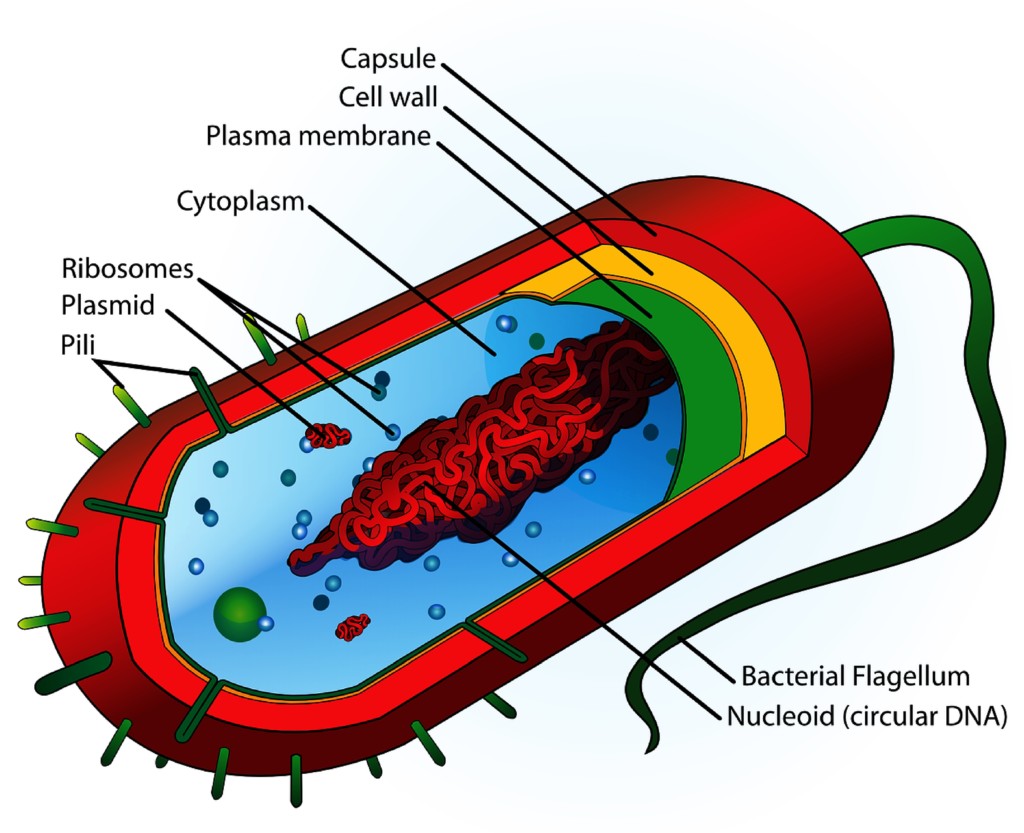
Most bacterial cells consist of layers forming what is called the “cell envelope”. The inside “layer” is the cytoplasm. A cell or plasma membrane encapsulates the cytoplasm, separating it from the outside layer. The outside layer is the cell wall, which surrounds and protects the entire cell. This is a highly simplified description of cell structure, but is sufficient for our discussion.
Alcohols are able to kill many types of bacteria on the molecular level. An alcohol molecule has one end that can ‘grab’ or bond with aqueous/watery substances and another end that can bond with lipid/fatty substances. When an ethanol molecule comes in contact with a bacterial cell, its water-loving end bonds with and disrupts the hydrophilic parts of the outer cell wall, weakening it. Simultaneously, its oil-loving end bonds with and disrupts the fatty parts of the cell wall and the cell membrane. This bonding makes them “leaky” (more soluble) so that both layers lose structural integrity and fall apart, essentially dissolving them. As these layers disintegrate, additional alcohol molecules then enter the cell where they denature proteins, causing the bacterium to die.
A similar process occurs with mold and fungal spores, but we won’t get into a deeper explanation of this. Suffice it to say the alcohol kills the spores, preventing their growth and reproduction.
Q: I want my essential oil containing product to stay mixed. Will alcohol help? What else can I use?
A: As school science class taught us, oil and water don’t mix, and this includes essential oils as well. They are polar opposites and repel one another. Therefore, if we want them to “mix” and stay together, we need something to help us do that. Depending on the product, we would need to choose from:
• Dispersants
• Emulsifiers
• Solubilizers
• Surfactants
People often use these terms interchangeably when talking about formulating water and essential oil based products. However, the terms are actually dissimilar and have different, distinct actions. Let’s briefly look at the differences in dispersing, emulsifying and solubilizing. Then we’ll discuss ethanol as it acts as a solubilizer in an essential oil spray.
- Diluting is a process of reducing or decreasing the concentration of a substance (i.e. – a solute or solution) by mixing with another substance (i.e. – a solvent or a diluent) using more solvent or diluent than solute/solution. In chemistry, we use the principle “Like dilutes like.” which simply means hydrophilic substances will dilute hydrophilic substances (water in orange juice), hydrophobic substances will dilute hydrophobic substances (olive oil in essential oil), gases will dilute gases (nitrogen in oxygen), etc.
- Dispersing is a temporary forced scattering of a substance into smaller parts and many directions. The substance doesn’t change, it just scatters temporarily. Think of a bottle of olive oil and vinegar salad dressing. Shaking the bottle momentarily disperses the two ingredients together, and then you can pour it on your salad. As soon as you set the bottle down, though, you’ll notice the oil and vinegar immediately begin separating into their separate layers again.
- Emulsifying is a process using a chemical substance (an emulsifier) and high-shear force/rotation to force a bond to form between oil and water molecules. High-speed blending in combination with an emulsifier forces oil components to “disperse” into tiny micro-droplets throughout the water component without changing the molecular structure and polarity of either.
Simultaneously, the emulsifier molecules stand in between oil molecules and water molecules to create a “link” and forcing them to ‘play nice’ together instead of repelling one another. These new “linked” molecules are held close together in what is known as an emulsion, with the emulsifier (aka ‘mediator’) at the center, yet all the substances will remain molecularly distinct. Mayonnaise is an example of an emulsion, as is a water-based body lotion. - Solubilizing a process of using one substance (a solvent) to “dissolve” another substance (a solute) to create a unified solution. This is true even if substance #2 normally has a molecular aversion to substance #1 because they have opposite polarities.
Solubilizing is the chemical process we want when making a spray with essential oils. Ethanol is used to solubilize (‘dissolve’) essential oils into water based sprays so they can be blended or diluted without separating. At proper concentration, ethanol “marries” essential oils and water together, so they become one homogeneous substance that can no longer be separated into two distinct substances. There can be no separation or ‘divorce’, they’re forced to stay together for good! (e.g. lavender essential oil when added to high-proof Everclear® to make a room spray can no longer turn back into the separate substances of Everclear® and lavender oil. They’re forced to stay together as one aromatic solution which can then be diluted with a water component. - Surfactants involve somewhat complicated discussion, and our goal is to make a simple room spray, so we’ll leave this term out of our discussion.
Q: I want to use ethanol to both preserve my spray and to keep the essential oils ‘mixed’. Exactly how do I do this?
 The amount of ethanol alcohol added to your product matters as well as the alcohol concentration (percentage or ‘proof’). The time allowed for essential oils to dissolve also plays a role. Some essential oils will readily dissolve, like very light and volatile citrus oils. Other oils take a bit longer while still fewer can stay in alcohol forever and never fully dissolve (for instance, very heavy/resinous oils like myrrh). This is why the concentration (percentage or proof) of the alcohol you choose is so important.
The amount of ethanol alcohol added to your product matters as well as the alcohol concentration (percentage or ‘proof’). The time allowed for essential oils to dissolve also plays a role. Some essential oils will readily dissolve, like very light and volatile citrus oils. Other oils take a bit longer while still fewer can stay in alcohol forever and never fully dissolve (for instance, very heavy/resinous oils like myrrh). This is why the concentration (percentage or proof) of the alcohol you choose is so important.
For effective solubilizing purposes, the alcohol used needs to contain 95-100% ethanol. So while many enthusiasts are told to “just buy the cheapest vodka in the liquor store to make your room spray”, this is incorrect. A cheap vodka usually is 80 or 100 proof or only 40-50% ethanol content and therefore will not properly solubilize essential oils. In the event that 190 proof (95%) grain alcohol is not available, one can use 151 proof (~75%), but it may not solubilize essential oils as easily.
To properly preserve your water-based spray: the alcohol used needs to have a minimum of 60% ethanol content. In other words, it must be at least 120 proof grain alcohol (proof=twice the percentage of the alcohol). While less proof grain alcohol can kill microbes on surfaces, the objective here is to prevent microbial growth in your spray, not to kill microbes on contact with a surface.
To hit both the targets, I typically recommend Everclear® or a comparable brand of grain alcohol that is 190 proof (95% ethanol). At minimum, you’d want to use 151 proof (75.5% ethanol) for solubilization purposes. However, depending on the viscosity and/or specific gravity of the essential oils in your formula, even 95% ethanol may not always dissolve thick essential oils completely. In those cases, Special Denatured Alcohol (SDA or perfumer’s alcohol) is another option. However, it may not be available to home users and has restrictions and requirements regarding purchase.
On top of that, the percentage of ethanol that’s part of the total of the spray formula needs to be a minimum of 20-30%. I recommend 25% because it’s an easy number to work with when doing the math in product formulating. We use percentages for a product formula because it allows for consistent reproduction and batch size adjustments. You can make any size batch you want by using simple multiplication to determine the amount needed of each ingredient.
For example, let’s start with a 4 oz. (~113 grams) spray bottle to make a master base. You would use:
• 1 oz (~28 grams) 190 proof/95% ethanol (25% of the whole formula)
• 3 oz (~85 grams) distilled water (75% of the whole formula)
(gram measurements have been rounded for ease of measuring)
Subtract the percentage of essential oil you want to use from the water. If making a spray with 3% total essential oils, your formula would be 25% ethanol, 72% distilled water, and 3% essential oil. Using percentages, you can make any size batch you want by using simple multiplication to determine the amount needed of each ingredient.
Again using 4 oz. of spray, the approximate measurements for the above formula would be as follows (weight measurements):
• 1 oz (~28 grams) ethanol (95%/190 proof)
• 2.88 oz (~82 grams) distilled water
• 0.12 oz (~3 grams) essential oil
Because I’m a formulator, I’m accustomed to weight measurements and use a scale. You can pick up an inexpensive kitchen scale on Amazon or many ‘big box’ stores. But if you don’t have one, here’s a formula for a lovely room, fabric freshening, and deodorizing spray using volume measurements.
Added Tips: Keep your measuring method consistent for all ingredients. Weigh ingredients for accuracy whenever possible, using a cheap kitchen scale which can be purchased on Amazon or in any kitchen store. Weight-based measurements (grams, milligrams, ounces) are preferred over volume measurements (teaspoons, tablespoons, cups), especially for smaller batches. I also recommend sanitizing your tools, containers (inside and out), work surfaces, etc. with 70% isopropyl (rubbing) alcohol before formulating to reduce the amount of microbes right from the beginning. Allow the surfaces and containers to dry completely before you start making your spray. It’s also a good idea to wear nitrile or similar food preparation gloves to limit any cross-contamination from your skin to the product and to limit your own skin exposure to the ingredients. Undiluted alcohol is not skin-friendly and it can cause dry skin or irritation. Undiluted essential oils on skin can result in adverse reactions.
**This same base formula can be used for do-it-yourself body sprays and personal fragrances.
Important to note:
- Damage to surfaces and finishes such as finished wood, countertops, or even manicures can occur with any alcohol, including ethanol. Remember, it’s a solvent and so are many essential oils. Take care to avoid direct contact with finished surfaces.
- Equally important, if making a body spray, make sure to avoid using phototoxic essential oils if you plan to go outdoors after use and observe general safety and safe dilution recommendations for all essential oils. Those are easily found in Essential Oil Safety, 2e (Tisserand & Young)
- If you plan to sell a handmade product containing ethanol, check all the corresponding federal, state and county/city laws, restrictions and requirements first. This gets into some sticky areas that could get you into serious trouble.
Conclusion
In conclusion, ethanol (ethyl alcohol) is a ‘cidal’ agent capable of killing microorganisms. It can be used to eliminate microbial contamination and prevent microbial growth. It is also a solvent capable of dissolving oils. As such, it’s suitable to use as a solubilizer when dissolving small amounts of volatile essential oils.
For the at-home DIY enthusiast making sprays, colognes, and similar products, using grain alcohol (ethanol) is a simple, cost effective way to safely prepare a water-based product such as a room spray. It’s kind of a “one-stop shop” in terms of both preserving and mixing, which means you don’t have to learn about more involved preservative systems. However, at least 60% (120 proof) alcohol is needed for preservation, and at least 75% (150 proof) for solubizing essential oils.
GLOSSARY OF TERMS
Microorganism – An organism that can be seen only with the aid of a microscope and that typically consists of only a single cell. Microorganisms include bacteria, protozoans, and certain algae and fungi.
Cytoplasm – The part of a cell between the cell membrane and the nuclear envelope, where the functions for cell expansion, growth, metabolism, and replication are carried out.
Plasma Membrane (syn. Cell Membrane) – A semi-permeable microscopic membrane consisting of two layers, made of a layer of phospholipids embedded with proteins that forms the external boundary and separates the contents of the cell from its outside environment, as well as regulates what enters and exits the cell.
Cell Wall – A rigid layer of polysaccharides lying outside the plasma membrane of the cells of plants, fungi, and bacteria. It’s function is to give the cell strength and structure, maintain its shape and to serve are a protective barrier and filter molecules that pass in and out of the cell.
Water-soluble – Capable of dissolving in water
Volatile – Evaporating rapidly; Passing off rapidly in the form of a vapor
Dispersant – a material or substance that breaks up another substance into smaller particles, distributing them throughout a medium in a random fashion to create a suspension. In product formulation, this usually applies to a dry powder such as pigments in a wet medium.
Emulsifier – a substance that is soluble in both fat and water which enables fat to be uniformly dispersed in extremely fine micro-droplets and then remain dispersed in water creating a homogeneous solution called an emulsion.
Solubilizer – a substance that increases the solubility and dissolvability of another substancein an otherwise incompatible medium, such as oil in water.
Surfactant – a substance or compound that reduces the surface tension (or interfacial tension) between 2 liquids or a liquid and a solid. The word “surfactant” is a contraction of the three words “Surface Active Agents”.
Polarity – a separation of electric charge that leads to a molecule having positive and/or a negative end which determines bonding capabilities.
‘Cidal‘ – Suffix indicating killing or capable of killing, as in bacteriocidal (capable of killing bacteria) and fungicidal (capable of killing fungi) and insecticidal (capable of killing insects).
Additional References and Information Sources
Preservative: https://en.wikipedia.org/wiki/Preservative
Why Cosmetics Need Preservatives: http://personalcaretruth.com/2010/06/why-cosmetics-need-preservatives/
Why Use A Preservative: http://sagescript.blogspot.com/2009/01/why-use-preservative.html
5 Reasons Why Your Natural Formulations Need Preserving: https://formulabotanica.com/5-reasons-why-your-natural-formulations-need-preserving/
Moisturizing body milk as a reservoir of Burkholderia cepacia: outbreak of nosocomial infection in a multidisciplinary intensive care unit: https://ccforum.biomedcentral.com/articles/10.1186/cc6778
When Should You Use A Preservative: http://swiftcraftymonkey.blogspot.com/2012/01/when-should-you-use-preservative.html
Preservative Information: http://www.cosmeticsinfo.org/preservative-information
Water Microbiology: http://science.jrank.org/pages/7311/Water-Microbiology.html
What Are Microbes? – http://www.edu.pe.ca/southernkings/microintro.htm
Where Do Microbes Live? – http://www.edu.pe.ca/southernkings/microhabitats.htm
Control of Microbial Growth: http://textbookofbacteriology.net/control_1.html
Structure and Function of Bacterial Cells – http://www.textbookofbacteriology.net/structure.html
Bacteria Cell Structure – https://micro.magnet.fsu.edu/cells/bacteriacell.html
The Bacterial Cell Envelope – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2857177/
Bacterial Cell Wall – http://www.pathwaymedicine.org/bacterial-cell-wall
Prokaryotic Cell Structure: The Cytoplasmic Membrane – http://faculty.ccbcmd.edu/courses/bio141/lecguide/unit1/prostruct/cm.html
Biology Study Flashcards – https://quizlet.com/31829880/biology-study-flash-cards/
Ethanol – What Is It? – https://web.extension.illinois.edu/ethanol/
What Is Ethanol? – Formula, Structure and Uses – http://study.com/academy/lesson/what-is-ethanol-formula-structure-uses.html
The Manufacture of Alcohols – http://www.chemguide.co.uk/organicprops/alcohols/manufacture.html
Antisepsis, Disinfection, and Sterilization – http://www.asse.org/assets/1/14/4420_chapter_4_sample%20pages.pdf
What Is Germicide? – http://www.wisegeekhealth.com/what-is-germicide.htm
How Does Ethyl Alcohol Kill Bacteria? – http://scienceline.ucsb.edu/getkey.php?key=2160



Can 91% rubbing alcohol be substituted? It’s much less expensive.
Hi Aimee, just checked this with Ginger and she doesn’t recommend it as it would behave differently and the percentages would have to be reworked, or it would have to be used straight without water.
Ginger, thank you so much!!! this is so timely and I have been looking for more information to no avail… I appreciate the detailed and specific information…
SO is the witch hazel with alcohol better than the one without or do you still need to do all this so it mixes right???
Hi, Paula. Thanks for reading and for your question. The alcohol in witch hazel is only sufficient to protect the witch hazel itself from some microbial growth. It will not effectively inhibit microbial growth in a spray or other product. In fact, it will provide food for microbes because it’s an herbal/botanical product/ingredient. Nor will it solubilize essential oils so they will just float in the liquid base. I do not recommend substituting witch hazel with or without alcohol in a spray in place of the ethanol as advised in the article or to replace of the water.
Can a mixture with Everclear be used on fabrics like clothes or furniture???
There should be no issues with fabrics when using a fine mist sprayer except possibly very delicate ones such as silk. But it’s always wise to test any substance on a hidden area of the fabric to make sure it takes the substance without fading or spotting.
Thank you for such an informative article and the additional links. Just the information I needed.
Wow, great blog! A short course in safety when DIY essential oils. This is a required reading.
I’m missing something. If we need 60% alcohol to preserve, why can we dilute to 25% for our finished product?
The ethanol alcohol content of the alcohol used (aka. consumable grain alcohol/liquor, diluted pure ethanol or special denatured alcohol) needs to be at least 60% ethanol for it to effective inhibit microbial growth in a product with water or water equivalent. 60% ethanol content would be 120 proof – 60% ethanol/40% water in the bottle of consumable grain alcohol. Then 20% or higher of the final total spray formula needs to be that 120 proof/60% ethanol content alcohol for it to be effective in the formula as a whole. I use the 25% for calculating the total per formula because it’s easy math calculations and adds a little buffer for protection.
Thank you so much for this great article, it was exactly what I was searching for with regards to how to start learning how to properly incorporate essential oils into a water-based product. And this comment answers the same exact question I still had after reading it 😉
So just to make sure I understand your answer, you saying that the 25% calculation is based on using an alcohol that is 120proof. So if I use an alcohol that is HIGHER than 120proof, then after I solubilize the essential oils, I could dilute the solution with water down to the equivalent of 120proof, and then use the suggested formula to add additional water at the appropriate ratio for a 120proof starting alcohol. So while I’m *technically* adding more total water in this scenario, the percentage of ethanol in the final product would be equivalent since I started with a higher proof.
Is that correct? Thank you again!
This is a great article! However in some states like California the high proof alcohols are almost impossible to find. What do you recommend if 100 proof is the highest we can access?
In this case, you could try to find and obtain perfumer’s alcohol (special denatured alcohol for perfumery) from a supplier. You could use straight 100 proof alcohol with no water added but the essential oils will probably not solubilize well and completely. It’s not potent enough to act as an adequate solvent for heavier, resinous or more viscous substances. Otherwise, you would need to invest in learning about safe product formulating, appropriate product preservation and all the necessary chemistry involved with formulating and products safe effective products.
Hello!
Great and very informative article. Thank you!!!
In my state, Everclear is banned, however, strangely, some liquors over 190 proof are allowed.
In any case, I purchased 192 proof Polmos Spirytus Rektyfikowany (96% volume and called a “rectified spirit” from Poland. FYI, in case you don’t know as I didn’t, a Rectified Spirit is, according to Wikipedia, “…also known as neutral spirits, rectified alcohol, or ethyl alcohol of agricultural origin is highly concentrated ethanol which has been purified by means of repeated distillation, a process that is called rectification.”).
I hope this will be a good replacement for the Everclear and, if so, maybe others here who cannot obtain Everclear in their state may find this instead.
All the best!
How long will a product last using Everclear & does it need to be refrigerated?
Following the instructions given, it should last for months with no concerns without refrigeration. If you wish to refrigerate, you can.
HI great info! thanks 🙂 how high is it possible to go on Essential oils and still have a stable solution?
Thank you for your comment and for sharing!
So I made a bug spray with 2 oz of everclear 190 proof and 2 oz of distilled water with 72 drops of EO. I mixed the EO with the everclear and shook it and let it sit for several hours. Then mixed with the water. And shook. But I still EO sitting at the top. Why is this? Did I do something wrong?
Hi Ina, You may try a few things. Mix the essential oils with the alcohol and let them sit for at least an hour (or longer, until they are fully mixed in to the alcohol. Make sure you slowly pour in the water. We offer an Aromatic Formulation course that will help you become more confident. We will offer it again in 2021. Make sure you are on our email list to receive updates. 🙂 ~Shane
https://tisserandinstitute.org/aromatic-formulation/
It depends on the essential oils used and safety protocols involved with each. There’s no hard and fast rule for air sprays but rarely would one need to go above 3-5% for an air or linen spray. For skin contact, safe dermal limits need to be followed to reduce risks. Also inhaled safety issues and those which carry contraindications by all routes should be observed.
Thank you thank you!! As a long time student of aromatherapy, and a licensed skincare professional, I am acutely aware of the necessity for preservatives. This is such a well written article, I am sharing it everywhere!
I appreciate this article, just wondering about the % of dilution though … If there’s 600 drops of EO per ounce and 6 drops would be a 1% dilution, then 24 drops would be 1% for 4 ounces and 24×3 = 72 drops for a 3% dilution in 4 ounces. How can 108 drops also be a 3% dilution?
Hi Rebecca, our formulations work with 900 drops per ounce, or 30 drops per ml, which more accurately reflects the average drop size (which varies between essential oils and droppers). For more info, see here Hana
Wow. This is so mUch more than i expected To find. Just doing a search for a rooM spray recipe and i feel like im in science class all over again. ThiS is so INformative for everyone. ThAnks so mUch for all youR Questions and Answers. I fEel so much wiser. KNOWLEDGE IS POWER AFTER ALL. Cheers from new zealand
Great information. I was wondering if Vodka can be substituted?
Hi Antoinette, it can, however it has to be high proof (at least 150 proof/75%).
Thanks Ginger, great information! It was not mentioned but I would think one would need to be careful when spraying this around open flames…candles, gas or propane stoves etc…
Hi Crystal. Normal precautions in this instance would be wise, yes. Both the alcohol and essential oils are volatile substances with flash points.
Thank you so much for the information. Would you use the same 25% of the total in a blend of oils in a carrier? Also, Is it necessary to add a preservative to a blend that are just oils, like for perfumes?
Hi Brenda,
If you only add essential oils to a carrier oil (creating an anhydrous, or water-free product), you don’t need to add a preservative. Preservatives come into play once water is present. When blending with vegetable oils you don’t need to add anything, unless you need to create a product with a long shelf life. But that is a subject for another blogpost. If you only use essential oils, there is no need for a preservative, either.
Thank you for your response. I have another question as I was reading it again. It mentions wearing gloves as the alcohol could irate the skin. But then mentions it being used as a body spray. I am confused on this.
Paula, I recommend wearing gloves to reduce the risks of contaminating the product with any debris or transferring microbes from the skin, around or under the nails, etc. into the product, onto the bottle or sprayer. I go on to say undiluted and barely diluted alcohol such as Everclear is hard on the skin and can cause dryness, especially with repeated or prolonged exposure. So wearing gloves helps protect your hands in the event of a spill or splashing. The finished product of a body spray will not contain undiluted alcohol. It will be heavily diluted with water. The final product will have only 1/4 of the total being alcohol and 3/4 minus the teaspoon will be distilled water.
Hi there,
When mixing essential oils into a carrier oil for perfume, would you still recommend adding alcohol so that it absorbs better and isn’t so oily?
Thanks in advance
Hi Natalie,
If you are creating a perfume, you pretty much have to choose either alcohol-based or oil-based formulating. A combination of vegetable oil and alcohol would not be practical.
Thanks Hana, much appreciated. One more question please, how do you get rid of cloudiness when making the room spray? Is that normal? Some products on the market that have both alcohol and water are totally clear. Is this because they use other chemicals to remove the cloudiness? Tia. Natslie
PERFECT ! Thank you for such an informative article and the additional links. Just the information I needed…. I’ve been doing research desiring to make room sprays… which becomes very confusing/conflicting info at times…appreciate
Thank you so much for this very informative article- it is EXACTLY what I was looking for!!!
Bingo! Finally found this after so much researching! Thank you
Hello!
I loved this article, so informative!!
I have a question though – would I be able to swap alcohol for salt for the rooms sprays?
Greetings, Salt would not be appropriate in this case. ~Shane
Thank you – finally very clear answers to most of my questions on room sprays! One more question though – I see some formulas online that use Witch Hazel instead of alcohol. What does Witch Hazel actually do? Thank you!
Great question! Witch Hazel is water based and would not be a substitute for alcohol. 🙂 ~Shane
Thank you so much for the info. I have been looking for a long time.
Thank you Ginger. Understanding the “chemistry of” Gives me insight to how the world around us works. The links in your article are a great resource and furthur my understanding even more. The knowledge you share is what I have been seeking but seems hard to find with out an organic chemistry background. Props!
hi ginger ty fpr your excellent work i am interested in the extraction of essential oils but am averse to oils made from isopropyl alcohol particularly those used for edible uses and consumption… isopropyl has a residual bitter taste and bottles have|” not for internal use “on them is this reasonable or is isopropyl totally acceptable for consumption after best efforts to remove residues ?
Hi Michael, the sprays made with essential oils and alcohol are not to be consumed. In general, essential oils themselves shouldn’t be casually consumed. There is a time and place for the oral and internal use of essential oils, with proper training. We cover all routes of use in our Essential Oil Safety program and we will offer it again in 2021. You may be interested to join us. ~Shane
https://tisserandinstitute.org/essential-oil-safety-master-class/
I see this is for water based solutions, but what about if you use essential oils mixed with a carrier oil. Is it ok to just have that in a roller bottle for various uses? Or does that also create a breeding ground for microbes? How long could it be kept in a rollerbottle?
Hi! this was useful. I was wondering if you are making an essential oil hydrosol spray for face/body which preservatives should I use, because I know that blending it with alcohol would not be a good idea, is vegetable glycerin good for it?? thanx a lot.
Hi. Sometime ago I made a Sleep Pillow Spray and the recipe said to use Everclear and Lavendar. I couldn’t remember what else but i followed the instructions clearly I did try to spray and it did really smell great and helped me to fall asleep. After having to leave my home to go up north to take care of my mom during the coronavirus crisis, I came back home and had wanted to use that pillow spray but I couldn’t spray anything out so I opened the bottle and was surprised to see that the long plastic tube inside the bottle had disappeared or should I say dissolved. What went wrong?
Essential oils will dissolve some plastics in sufficient concentration, though it seems odd if The Lavender oil was dissolved in the alcohol. Perhaps un unsafe formulation?
Hi, I love this article as everyone else in this thread. If I need my end product(air freshener) to have 60% alcohol, as recommended to work as a preservative, how much of the 95% Ethanol/190 Proof Everclear should I use? Final product would be 8 ounces.
Thank you so much!
Hi Andres, We offer a single class called Essential Calculation that helps you walk through calculations and conversions for formulating. This may be of interest to you. 🙂
https://tisserandinstitute.org/calculation/
Can I rely on 190-200 proof alcohol to kill all…bacteria, parasites etc in plant materials used for herbal tinctures?
Hi please, can you tell me what is the best formula for a home fragrance used for a diffuser?
Hi Maha, Many essential oils are safe to use in a diffuser. The ideal aroma is going to be subjective based on the person diffusing. You may enjoy our Essential Oil Safety masterclass where we cover all routes of use and the most current safety information regarding essential oils. ~Shane (shane@tisserandinstitute.org)
Essential Oil Safety Masterclass: https://tisserandinstitute.org/essential-oil-safety-master-class/
Hi Ginger, this article was so insightful thank you. I kept seeing so many blogs with recipes for EO sprays that Didn’t include alcohol. So, It was great to find a reliable source!
HELLO, THANK YOU FOR THE INFORMATIVE POST. IT IS MY FIRST TIME TRYING TO USE ALCOHOL TO SOLUBILIZE MY ESSENTIAL OILS INTO A WATER-BASED GEL FORMULATION. IF I WILL USE A SEPARATE PRESERVATIVE, WILL I Still need to use that much alcohol in the formula?
If not, what is the ideal alcohol:essential oil ratio that you can recommend?
Hi Christine, There are alot of factors involved, especially when using preservatives. You might consult with a formulator, or look into our Aromatic Formulation programs in 2022. We do have a level focused on Gels & Serums. 🙂 ~Shane Carper
Hi,
Is perfumers alcohol suitable/safe for making sprays?
Thanks
If you need alcohol, then yes this is a good choice.
Great article! I’m wondering if the measurements Are different if using frAgrance oIl vs essential oil, Given the synthetic properties?
Thank you Katie, and no the measurements would not change for a fragrance oil.