
Fall and winter usher in the season of acute respiratory illnesses, and many people are beginning to suffer from sneezing, sore throats, coughs, fever, and achiness. There is often confusion surrounding the reason for these ailments, and common terms like “cold”, “flu”, “pneumonia”, and “stomach flu” are used to describe distinct bacterial, viral, or fungal infections (Table 1). Proper diagnoses are key to efficient treatment, because treatments for infections are very different depending on whether the infection is caused by bacteria, viruses, or fungi. In some cases, coinfections (both bacterial and viral) can further complicate diagnosis. Respiratory syncytial virus (RSV) and the bacterium Haemophilus influenzae, and influenza and the bacterium Streptococcus pneumoniae are found together in children suffering from respiratory infections (O’Grady et al 2016), requiring multiple treatments to resolve the infection.
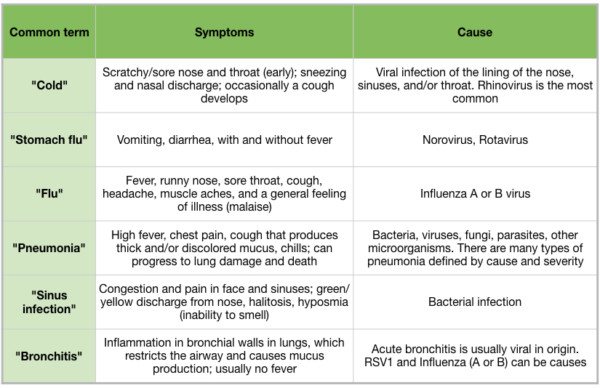
Table 1
Although influenza viruses have not been as deadly in recent decades as in the past, it is still vital to look for new ways of combating flu epidemics. The development of treatments that target multiple steps in the Influenza A Virus (IAV) life cycle will be the most effective way to fight IAV infection, clinical complications, and prevent IAV spread. Essential oils and their constituents have been shown to be effective antibacterials (Tisserand 2015), and clarification of their action as antivirals is crucial. Treatments with essential oils and other aromatics as an adjunct to allopathic medicines have been helpful to alleviate influenza symptoms such as malaise, headache, and runny nose (Price and Price 2007). However, there is evidence that essential oils can take an active role in reducing influenza complications and spread as well. This review will describe the influenza life cycle, its interaction in the host cell, and promising research that suggests aromatic compounds are effective treatments for influenza infection and prevention of influenza spread.
Influenza overview and history of pandemics
Influenza viruses are classified as type A, B, or C by the virus’s hemagglutinin (HA) and neuraminidase (NA) proteins, and are named with the presumed place of origin. Influenza A and B cause the typical “flu” symptoms, but influenza C is typically not a concern for human health. While influenza can sometimes be fatal, in general, influenza deaths are due to complications of the infection, including lung damage, pneumonia, and secondary bacterial infections. Patients with a high risk of complications from an influenza infection include young children, adults over 65, pregnant women, and those with chronic health conditions affecting the respiratory and circulatory systems. These patients generally develop influenza-induced pneumonia, secondary bacterial pneumonia, or acute respiratory distress syndrome, which can lead to death (Tesini 2018).
There have been six major influenza pandemics (worldwide outbreaks) since 1889. The 1918 influenza pandemic (“Spanish flu”) was the deadliest pandemic in history. Approximately 5% of the world’s population was infected, and the number of deaths has been estimated at 50 million (CDC). This strain of H1N1 caused the highest mortality because of secondary bacterial pneumonia (Shanks 2015) and cytokine storm (Wang et al 2018), rather than because of influenza-induced pneumonia or lung damage. A novel version of H1N1 influenza was responsible for the “swine flu” pandemic in 2009 (Tesini 2018, CDC). The Centers for Disease Control (CDC) estimates that 60.8 million people were infected in the first year of the pandemic, and between 151,700 and 575,400 people died (CDC). With the introduction of influenza vaccinations and pharmaceutical medications, the number of influenza deaths due to complications of influenza infection have fallen (CDC).
Antiviral drugs
Antiviral medications have largely been developed to prevent the virus from entering the host cell and unloading its viral genome, and also preventing viral replication or the viral progeny from exiting the host cell. Drugs that inhibit viral DNA (deoxyribonucleic acid) polymerase enzymes, such as acyclovir and ganciclovir, are effective treatments for herpes simplex virus (HSV) and cytomegalovirus, respectively (Gnann et al 1983, Matthews and Boehme 1988). However, DNA polymerase inhibitors would be ineffective to prevent genome replication in viruses whose genomes are organized as RNA (ribonucleic acid), such as influenza and hepatitis C. As such, the term “antiviral” does not mean a particular substance will kill all viruses. Instead, an antiviral compound may be virus-specific, or act on similar viruses.
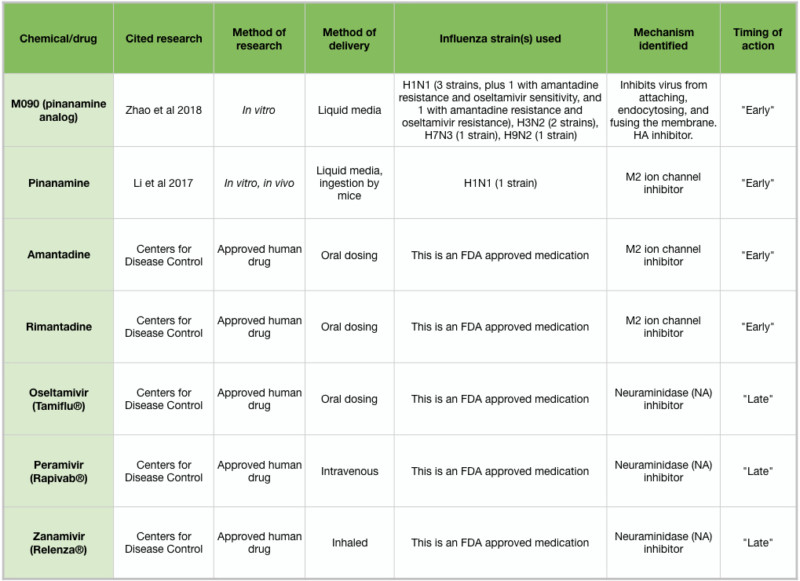
Table 2: Overview of antiviral drugs
Drugs currently approved to treat influenza infections include amantadine and rimantadine, which target the early stages of influenza infection, and oseltamivir (Tamiflu ®), peramivir (Rapivab ®), and zanamivir (Relenza ®), which target the late stages and spread of influenza. These medications must be administered within 1-2 days of symptom onset (Tesini 2018). Unfortunately, many strains of influenza have developed immunity to the available medications, and so there is a pressing need to develop new treatments. Two new chemicals, pinanamine and M090, have been shown to effectively block NA activity and influenza spread (Figure 2J), and may become approved human drugs following clinical studies (Li et al 2017, Zhao et al 2018). Because of the chemical complexity of essential oils and other aromatic compounds, IAV strains may be less likely to develop resistance. However, if the antiviral action of an essential oil is due to a particular chemical constituent, IAV strains may arise that are resistant to that chemical. Further in vitro testing may clarify this issue.
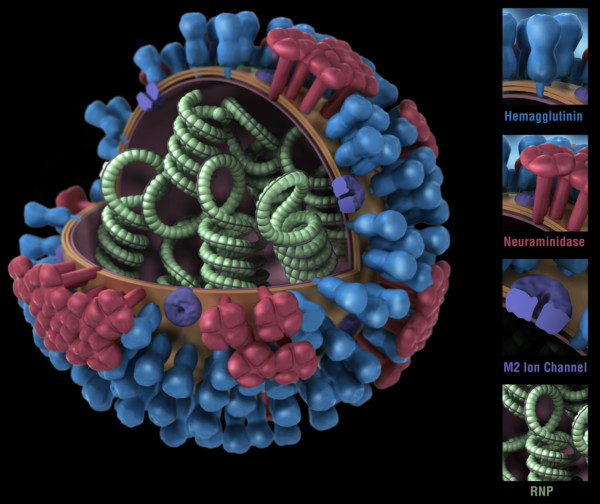
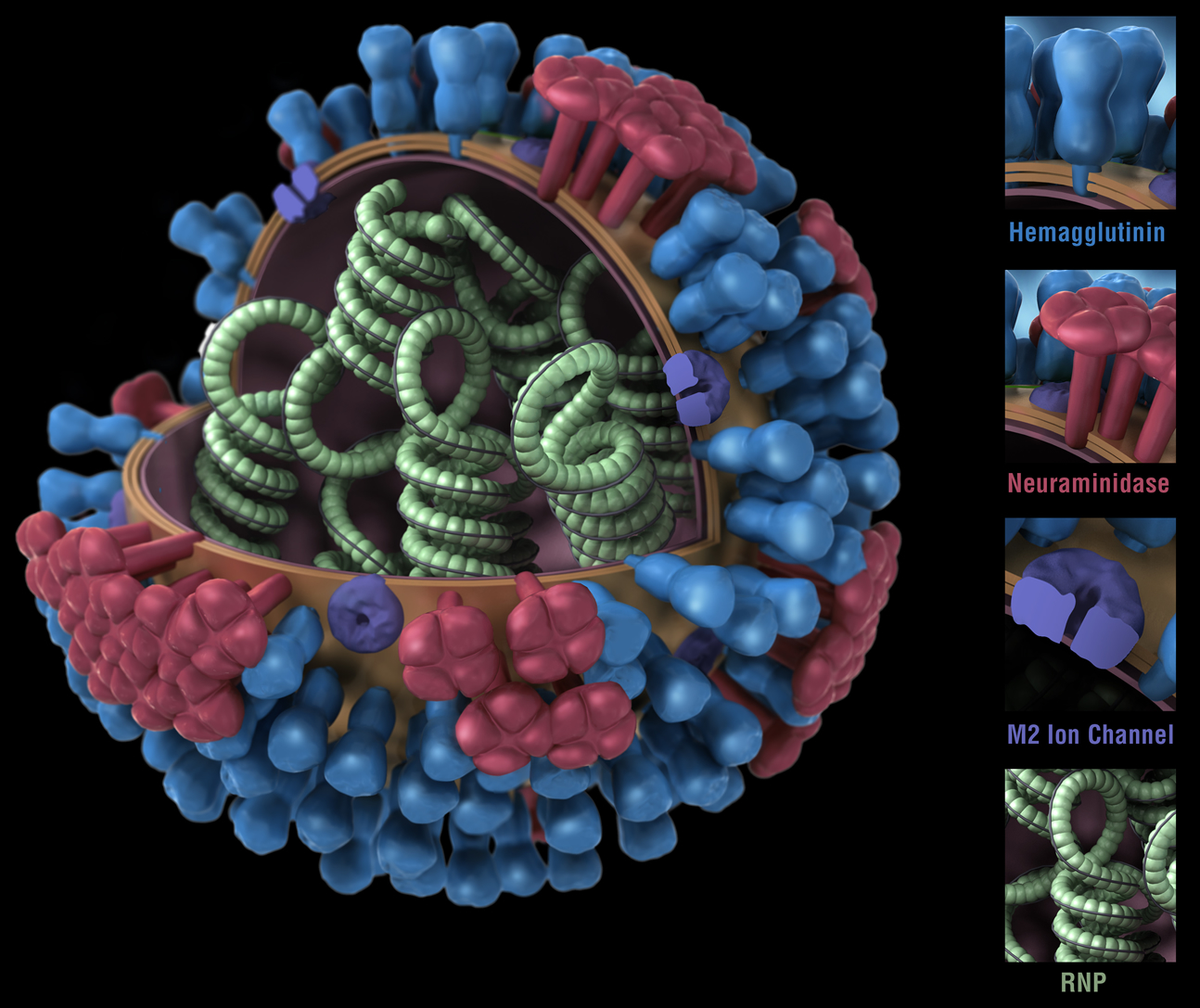
Figure 1. Influenza virus. RNP: ribonuclear protein. CDC
The biology of influenza
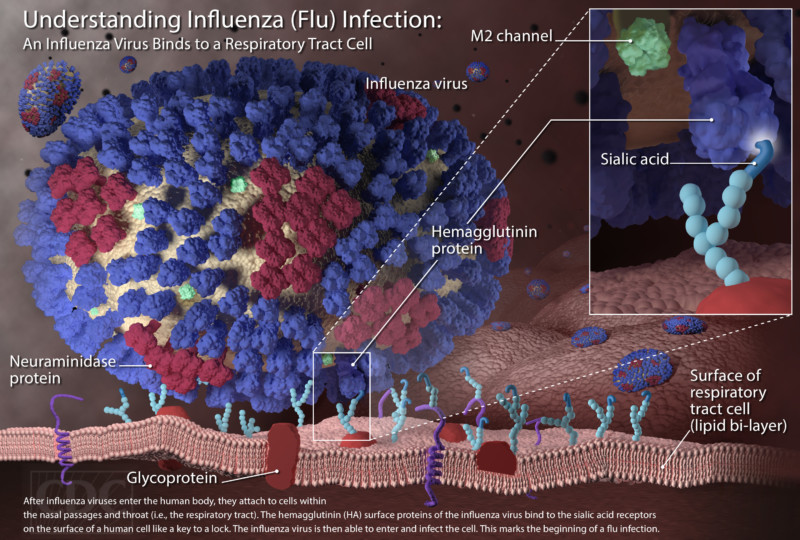
While bacteria are single-cell organisms, viruses are much simpler, being made of genetic material in the form of single- or double-stranded DNA or RNA, plus a protein coat called a capsid. They cannot reproduce or spread without invading a host cell. Coated viruses such as influenza, HSV-1, HIV, Ebola, and Zika have an additional coat around the capsid, called the envelope. The envelope consists of a membrane made of phospholipids that are usually derived from the host cell upon exit, and glycoproteins, which are derived from the viral replication process. Influenza A virus (IAV) is usually shaped like a sphere, and possesses three types of transmembrane proteins: hemagglutinin (HA), neuraminidase (NA), and matrix-2 proton channel (M2) (Samji 2009). All of these play a role in the life-cycle of a virus in human cell.
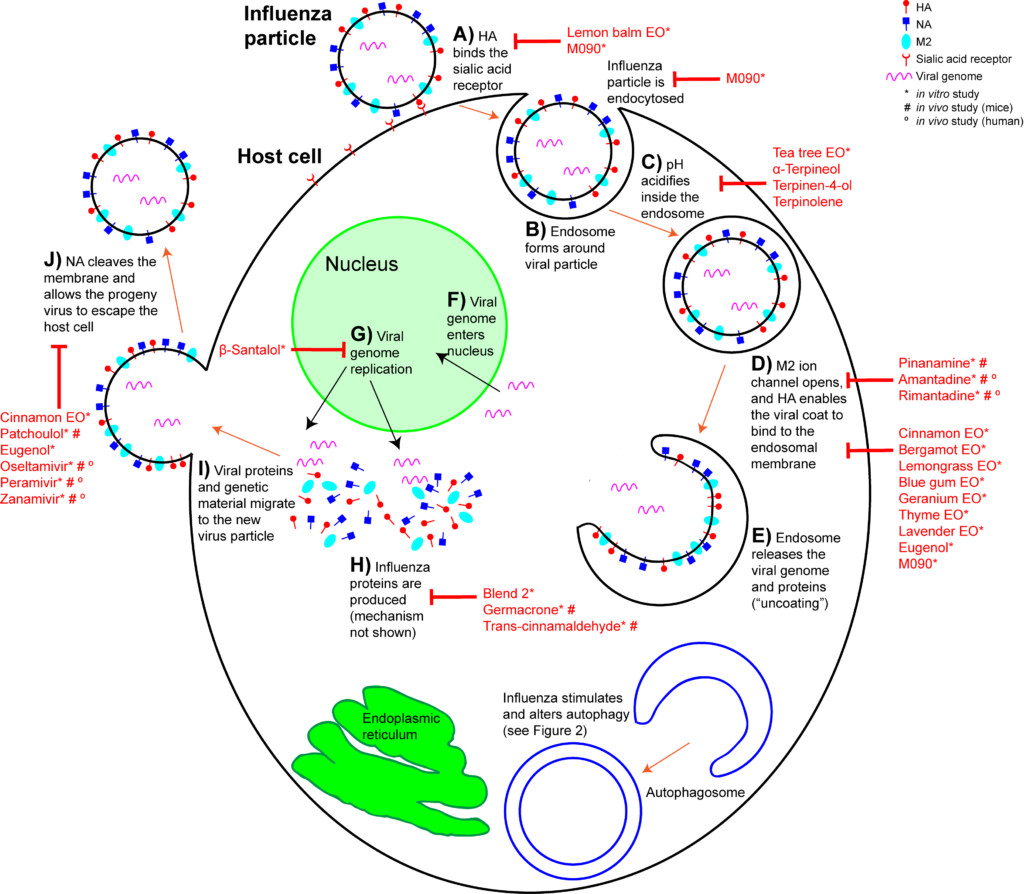
When an IAV particle encounters a host cell (typically epithelial cells in the nose, throat, and lungs), HA proteins bind sialic receptors found in the host cell’s cellular membrane (Figure 2A). This causes the host cell to endocytose the viral particle, forming a structure called an endosome (Figure 2B). Upon entry into a cell, coated viruses must undergo an uncoating process to remove the additional membrane, before the virus is capable of replication. Uncoating begins as the pH of the endosome acidifies, which triggers the M2 ion channels to open (Figure 2C). As this is happening, the receptor-bound HA undergoes a conformational change that allows the viral envelope to fuse to the endosomal membrane (Figure 2D). As a result, the IAV genome is released into the cytoplasm of the host cell (Figure 2E) (Samji 2009).
The IAV genome enters the nucleus, and undergoes replication using the host cell’s machinery, before being returned to the cytoplasm (Figure 2F, G). Following IAV protein production, these new components migrate to the host cell’s cellular membrane and begin to form progeny IAV particles (Figure 2H, I). In order to fully exit the host cell, NA proteins in the developing IAV particle cleave the cell membrane (Figure 2J). The progeny virus particles are then able to invade other cells.
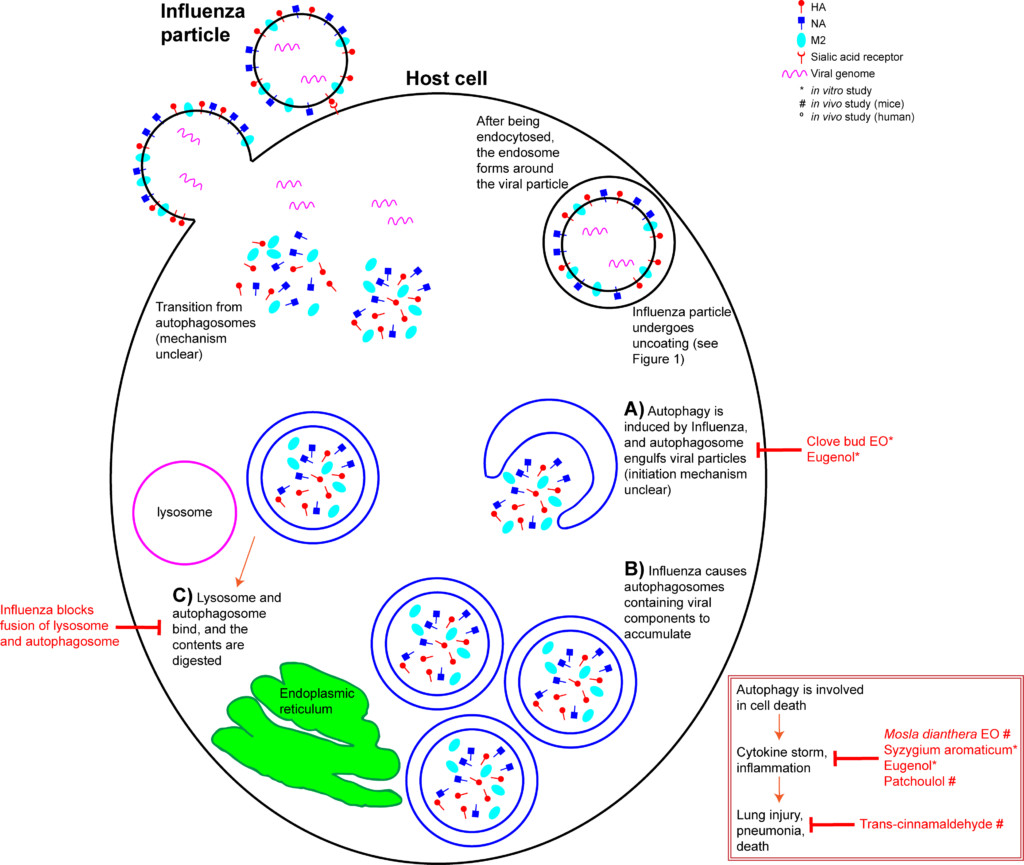
The host’s innate immunity is provoked upon IAV infection, and a cascade of inflammatory pathways are initiated. This manifests as flu symptoms. In addition, a process called autophagy is triggered within the host cell. Autophagy is a mechanism that recycles cellular content. It can be triggered when a cell is stressed, or when a cell detects damaged proteins that need to be degraded (Jackson 2015). Briefly, an autophagosome forms in the cytoplasm, engulfs the cytoplasmic contents, and becomes a double layer organelle (Figure 3A). The autophagosome then matures and fuses to the lysosome, where the contents are digested. Autophagy is involved in cell death and interacts with inflammatory systems (Wang et al 2018). In layman’s terms, autophagy means a cell “eating itself”, or anything it no longer needs.
Unfortunately, even this “self-cleaning” process can be hijacked by the Influenza virus. IAV M2 protein is involved in the induction of autophagy, and IAV takes advantage of the autophagy pathway to replicate (Zhang et al 2014, Jackson 2015). Autophagy is a critical for the accumulation of progeny viral components and alterations to the host cell’s apoptotic mechanism (Zhang et al 2014, Feizi et al 2017). IAV infection increases the number of autophagosomes in a cell (Zhang et al 2014) by inhibiting their maturation (Figure 3B) (Wang et al 2018). IAV also inhibits the fusion of autophagosomes to lysosomes (Figure 3C), which prevents degradation of its contents (Feizi et al 2017, Wang et al 2018). In effect, IAV hijacks the host cell’s autophagosomes to hide from the cell’s defenses. H5N1-induced autophagy is responsible for the IAV strain’s acute lung damage and high mortality rate (Wang et al 2018).
Additionally, IAV-induced autophagy leads to an increase in levels of chemokine and cytokine proinflammatory molecules (Figure 2 inset) (Feizi et al 2017). “Cytokine storm” describes this mass release of cytokines, leading to acute inflammation that starts in one location, but quickly spreads throughout the body, resulting in systemic sepsis (Tisoncik et al 2012). Cytokine storms due to IAV infection are thought to be the primary cause of death in H1N1 patients (Wang et al 2018).
In short, Influenza A Virus enters a cell, and uses its own mechanisms to replicate and to hide from the immune system. It is therefore vital to try and address a viral infection at all stages of development, and the in vitro research seems to show that some essential oils can do just that.
Aromatic compounds with antiviral activity
Most of the evidence for aromatic compounds being active against influenza is done in vitro, with cultured cells that are infected with IAV strains. These studies are ideal for determining the chemical and biological interactions within a cell. It is more straightforward to isolate specific points in the viral life cycle, and determine when a compound is interacting with cellular processes. However, translating in vitro studies to clinical applications requires in vivo studies, using model organisms. These studies are available for some aromatic compounds, but additional in vivo studies are necessary before advancing to human clinical studies. Compounds that are identified as inhibitors of specific points in the IAV life cycle in in vitro studies can be investigated as potential treatments in human patients. Essentially, although there is some promising research, the suggestions here are very tentative.
Prevention of migration, and early stages of IAV life cycle in a host cell
During the early stages of the influenza life cycle, the virus attaches to the cell, is internalized, and undergoes uncoating (Figure 2A-E). The approved drugs that treat early stages of IAV act on the uncoating process, specifically the opening of the M2 ion channel (Figure 2D). However, these drugs are ineffective for the majority of influenza cases now, due to mutations that cause drug resistance. Many aromatic compounds show anti-influenza action earlier in the infection process (Figure 2A-C). Lemon balm (Melissa officinalis) essential oil has been used extensively to prevent HSV infection. Likewise, IAV pretreated with lemon balm essential oil was unable to attach to host cell surface cell receptors in vitro (Pourghanburi et al 2016).
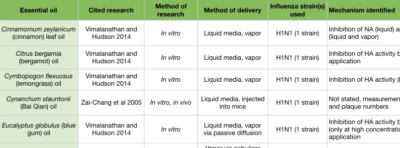
Tea tree (Melaleuca alternifolia) essential oil inhibits influenza during the early stages by preventing intracellular processing of the viral particle. When introduced into cell culture media, tea tree oil prevented viral uncoating by interfering with the acidification of the endosomes and membrane fusion (Figure 2C, D) (Garozzo et al 2009, Garozzo et al 2011, Garozzo et al 2013, Li et al 2013). The ability to prevent endosome acidification was ascribed to the tea tree oil constituents terpinen-4-ol, α-terpineol and terpinolene (Garozzo et al 2009, Garozzo et al 2011). These were tested against additional viruses, but their action may be IAV-specific. Tea tree oil, when actively diffused with a nebulizer for two seconds, cleared nearly all airborne IAV at 10 minutes, and showed zero virus at 15 minutes post nebulizer treatment (Usachev et al 2013). Blue mallee (Eucalyptus polybractea) oil showed zero virus at 15 minutes following a 15 second period of active diffusion with a nebulizer (Table 3) (Usachev et al 2013).
In an in vitro study examining H1N1, a number of essential oils and chemical constituents showed the ability to inhibit HA activity (Figure 2D). Cinnamon leaf (Cinnamomum zeylanicum), bergamot (Citrus bergamia), lemongrass (Cymbopogon flexuosus), blue gum (Eucalyptus globulus), and thyme (Thymus vulgaris) oils, when applied to the culture media, showed significant HA inhibition. Eugenol, a major constituent of clove bud (Syzygium aromaticum) and cinnamon leaf oils (Tisserand and Young 2014), was also an effective HA inhibitor (Vimalanathan and Hudson 2014). Lavender (Lavandula angustifolia) and geranium (Pelargonium graveolens) oils also inhibited HA, but only when used at high concentrations. When passively diffused for 10 minutes, bergamot and blue gum oils showed significant reduction in viral growth. When passively diffused for 30 minutes, cinnamon leaf and geranium oils showed a reduction in viral growth (Vimalanathan and Hudson 2014). However, these studies involved diffusing in small spaces (a small tube, and a closed chamber), and so room diffusion in a home may not achieve comparable results (Usachev et al 2013, Vimalanathan and Hudson 2014).
Middle stage of IAV life cycle in a host cell
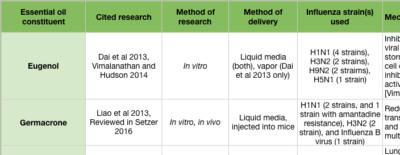
The “mid stage” of the influenza life cycle includes autophagy induction, IAV genome replication, and IAV protein production. Autophagy was effectively inhibited by clove bud oil and eugenol in cells infected with 8 separate IAV strains (Figure 3A) (Dai et al 2013). Markers for inflammation and cytokine levels were reduced compared to controls, and both viral replication and cell death was reduced (Figure 3 inset) (Dai et al 2013). When ingested by mice daily for 5 days after infection, Mosla dianthera essential oil reduced HA activity, and prevented lung inflammation and damage. This was likely due to improvements in the host’s immune system, as measured by cytokine levels (Wu et al 2012). (Mosla dianthera oil is not commercially produced. Its major constituents are elemicin 16.5%, thymol 14.5% and β-caryophyllene 14.5%.) When ingested by mice daily for 7 days, patchoulol prevented IAV-induced lung inflammation and damage. This was due to improvement in host immunity, as measured by T cell activation and cytokine/chemokine levels (Li et al 2012). Patchoulol is found in patchouli (Pogostemon cablin) oil at about 30%.
Following genome replication, IAV proteins are produced in the cytoplasm. β-santalol showed a reduction in IAV growth, and IAV genome synthesis in vitro (Figure 2G) (Kiyohara et al 2012, Paulpandi et al 2012). Germacrone effectively reduced IAV growth as well, and specifically reduced the transcription of viral genes and protein produced in vitro (Figure 2H) (Liao et al 2013). Germacrone showed an additive effect with oseltamivir (an NA inhibitor) in vitro and in vivo, suggesting germacrone may be an effective treatment when used in conjunction with traditional treatments (Liao et al 2013). Germacrone is found in zdravetz (Geranium macrorrhizum) oil at about 45%.
trans-Cinnamaldehyde, which constitutes about 80% of cassia (Cinnamomum cassia) oil and 70% of cinnamon bark oil (Tisserand and Young 2014), inhibited IAV protein production in vitro (Figure 2H) (Hayashi et al 2007). More importantly, intranasal application (when you introduce the liquid compound into the nasal cavity using a pipette) of trans-cinnamaldehyde, which enables direct inhalation of the vapor, was effective to treat lethal influenza-induced pneumonia (Figure 3 inset) (Hayashi et al 2007). This treatment could translate directly to humans, but because trans-cinnamaldehyde can be a skin sensitizer (Tisserand and Young 2014), inhalation without direct nasal application may be a better choice. The efficacy of an inhaled treatment would need to be confirmed in human trials.
Two essential oil blends have been shown to inhibit H1N1 growth in vitro (Table 5). Both blends contain cinnamon bark, blue gum, and rosemary (Rosmarinus officinalis) essential oils (Brochot et al 2016). Blend 2 additionally contains clove bud and sweet orange (Citrus sinensis) essential oils, and this blend inhibited viral protein production (Figure 2H) (Wu et al 2010).
Late stage of IAV life cycle in a host cell
In the “late stage” of influenza infections, viral proteins and genetic material migrate to the host cell membrane, and become a budding progeny virus (Figure 2I). In order for the progeny virus to escape the host cell and spread, NA must cleave the shared membrane. Cinnamon leaf oil and eugenol both inhibit NA activity in H1N1 exposed cells (Vimalanathan et al 2014), which inhibits the spread of IAV. Patchoulol, in addition to modulating a cell’s inflammatory response to influenza, is able to bind the active site on the NA protein (Wu et al 2011). Current NA inhibitors work on the same principle, and block the spread of influenza (Figure 2J).
Aromatic treatments suggested for home users
Influenza particles can spread by airborne droplets, close contact with an infected person, and contact with objects contaminated with the virus (Tesini 2018). Influenza particles can remain viable on objects for up to 48 hours (CDC), so effective sterilization of objects is an important step in preventing the spread of influenza. Current CDC guidelines suggest disinfecting objects with chlorine, hydrogen peroxide, soap, and alcohols (CDC). Ethanol is effective against most clinically relevant viruses, including influenza (Kampf 2017). Hydrogen peroxide has been shown to be effective against influenza and norovirus, when used on solid surfaces (Goyal et al. 2014). However, the efficacy of using these substances on porous surfaces is unclear. The development of chemicals that would be universal surface disinfectants is key to killing viruses in all settings. The use of essential oils to kill bacteria is established (Nazzaro et al 2013, Vasconcelos et al 2018), and evidence for their use to kill viruses is mounting. There is evidence that diffusion of essential oils is effective in eliminating airborne bacteria and viruses, but the safety of this practice must be explored.
Based on the in vitro study, nebulizing blue mallee (Eucalyptus polybractea) essential oil or tea tree (Melaleuca alternifolia) essential oils for 15 seconds is a fast and effective method to eliminate airborne influenza droplets within 15 minutes (Usachev et al 2013). These essential oils are considered safe for use with most people, and do not contain chemical constituents that are membrane irritants. However, most home users own diffusers with moderate output, rather than a commercial nebulizer. This may limit implementation of this method of killing airborne influenza. To increase efficacy, users could use a consumer nebulizer in a confined space, such as a closed bedroom. Following each treatment, the room should be aired out by opening a window to allow residual essential oils to leave, and to get fresh air into the room.
Passive diffusion of bergamot (Citrus bergamia) or blue gum (Eucalyptus globulus) essential oils for 10 minutes, or cinnamon (Cinnamomum zeylanicum) essential oil for 30 minutes, was enough to reduce influenza activity in cell culture. Blend #2, a commercial germ blend containing cinnamon bark, clove bud (Syzygium aromaticum), sweet orange (Citrus sinensis), blue gum, and rosemary (Rosmarinus officinalis) oils, effectively reduced influenza activity at multiple time points in cell culture (Table 5) (Wu et al 2010). This adds support for the practice of diffusing germ blends to sanitize rooms. Clove bud and cinnamon leaf oils are high in eugenol, which also showed extensive anti-influenza activity in mid-late stages of the influenza life cycle. Because cinnamon bark oil contains trans-cinnamaldehyde, an irritant and sensitizer, it is advisable not to use it in diffuser blends for an extended time period. However, diffusing for 30 minutes in an empty room, followed by airing out the room would be a reasonable practice. For the home user, an inhaler containing anti-influenza essential oils would be another option. Inhalers are great for on-the-go use, and eliminate the need to consider other people’s health conditions.
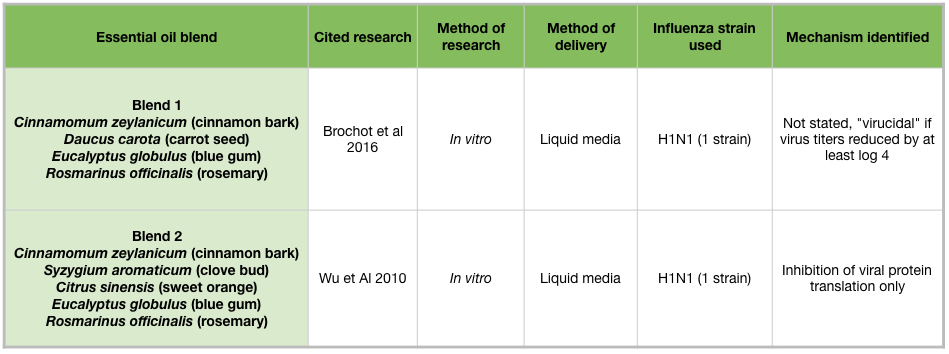
Table 5: Essential oil blends and influenza viruses.
Many essential oils were able to reduce influenza activity when applied directly to influenza- infected cells. Home users could use these in vitro results to determine the essential oils to use in cleaning surfaces. However, cleaning solid surfaces with hydrogen peroxide is likely a more effective and safer practice. It remains to be determined whether nebulized or diffused essential oils would be effective to clean porous surfaces. It is unclear how long influenza can survive on porous surfaces, and when possible, cleaning porous surfaces with CDC recommended substances is best.
Conclusions
Seasonal and pandemic influenza is a major concern for human health. Most influenza strains have become resistant to the available influenza medications, and there is a pressing need to develop new treatments. It is unclear whether influenza can develop resistance to complex chemical mixtures like essential oils, but it may be possible that influenza strains can develop resistance to particular chemical constituents such as trans-cinnamaldehyde. However, since essential oils and their components often act in multiple steps of the viral life cycle, resistance may be incomplete and only affect particular steps in the life cycle.
Numerous in vitro studies have clarified the mechanisms through which certain essential oils and their chemical constituents block influenza activity. These essential oils could be used to reduce influenza spread, but the next steps to develop aromatic therapies as influenza treatments is to conduct in vivo studies. Studies in mice have identified specific aromatic compounds that address the consequences of influenza including lung inflammation, lung damage, and pneumonia. Hopefully, these in vivo results will also lead to successful human treatments.
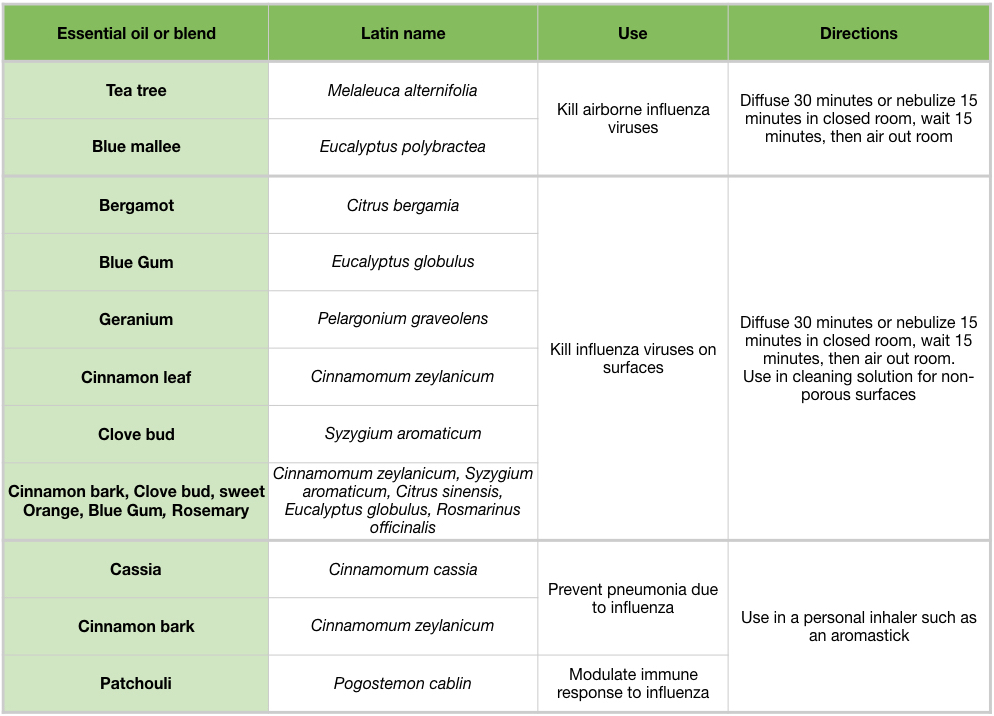
Table 6: Overview of essential oils and their effects and potential uses agains influenza viruses
Used terms |
Definition |
|---|---|
| Apoptosis | programmed cell death |
| Autophagosome | organelle that forms around cellular material to be recycled |
| Autophagy | mechanism that coordinates with lysosomes to recycle damaged or foreign material within a cell; usually can engulf larger material than lysosomes |
| Chemokine | a type of cytokine that is involved in infection |
| Cytokine | substance that is secreted by a cell that has an effect on another cell |
| Cytoplasm | intracellular space |
| DNA | deoxyribonucleic acid |
| DNA polymerase | enzyme that is involved in DNA replication |
| Glycoprotein | protein with and attached carbohydrate |
| Hemagglutinin (HA) | one of the type of proteins on the surface of influenza particles |
| HIV | Human immunodeficiency virus |
| HSV | Herpes simplex virus |
| Innate immunity | cells and mechanisms that provide the first line of defence from infection in a non-specific manner |
| In vitro | research done in cell culture (outside of an organism) |
| In vivo | research done in an organism; typically non-human |
| IAV | influenza A virus |
| Lysosome | a cellular organelle involved in degradation, digests intracellular material to recycle it |
| Matrix 2 (M2) | ion channel protein on the surface of influenza particles |
| Neuraminidase (NA) | one of the type of proteins on the surface of influenza particles |
| Pandemic | world-wide disease outbreak |
| Phospholipids | lipids containing phosphate that make up the membranes of many cells and organelles |
| RNA | ribonucleic acid |
| RSV | respiratory syncytial virus |
| Transmembrane | spanning a membrane; being outside of the cell, in the membrane, and inside the cell |
| Uncoating | process that removes the viral envelope |
| Viral capsids | protein coat surrounding viral genome |
| Viral envelope | phospholipid coat containing viral proteins that surrounds some viral capsids |
References
“Cleaning to Prevent the Flu.” CDC, https://www.cdc.gov/immigrantrefugeehealth/pdf/seasonal-flu/contamination_cleaning_english_508.pdf. Accessed October 12, 2018.
“Past Pandemics.” CDC, https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html. Accessed October 29, 2018.
“Quadrivalent Influenza Vaccine.” CDC, https://www.cdc.gov/flu/protect/vaccine/quadrivalent.htm. Accessed October 12, 2018.
Arora, Rajesh, et al. “Potential of Complementary and Alternative Medicine in Preventive Management of Novel H1N1 Flu (Swine Flu) Pandemic: Thwarting Potential Disasters in the Bud.” Evidence-Based Complementary and Alternative Medicine, vol. 2011, pp. 1-16. doi:10.1155/2011/586506.
Brochot, Amandine, et al. “Antibacterial, antifungal, and antiviral effects of three essential oil blends.” MicrobiologyOpen, vol. 6, no. 4, 2016, pp. 1–6., doi:10.1002/mbo3.459.
Dai, Jian-Ping, et al. “Drug Screening for Autophagy Inhibitors Based on the Dissociation of Beclin1-Bcl2 Complex Using BiFC Technique and Mechanism of Eugenol on Anti-Influenza A Virus Activity.” PLoS ONE, vol. 8, no. 4, 2013, pp. 1–16., doi:10.1371/journal.pone.0061026.
Feizi, Neda, et al. “Autophagy induction regulates influenza virus replication in a time-dependent manner.” Journal of Medical Microbiology, vol. 66, 2017, pp. 536-541. doi:10.1099/jmm.0.000455.
Garozzo, A, et al. “In vitro antiviral activity of Melaleuca alternifolia essential oil.” Letters in Applied Microbiology, vol. 49, 2009, pp. 806-808. doi:10.1111/j.1472-765X.2009.02740.x
Garozzo, A, et al. “Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: study on the mechanism of action.” Antiviral Research, vol. 89, 2011, pp. 83-88. doi:10.1016/j.antiviral.2010.11.010
Gnann, JW Jr, et al. “Acyclovir: mechanism of action, pharmacokinetics, safety and clinical applications.“ Pharmacotherapy, vol. 3, no. 5, 1983, pp. 275-283.
Goyal, SM, et al. “Evaluating the virucidal efficacy of hydrogen peroxide vapour.” Journal of Hospital Infections, vol. 86, no. 4, 2014, pp. 255-259. doi:10.1016/j.jhin.2014.02.003
Greber, UF, et al. “Mechanisms of virus uncoating.” Trends in Microbiology, vol. 2, no. 2, 1994, pp. 52-56.
Hayashi, K, et al. “Inhibitory effect of cinnamaldehyde, derived from Cinnamomi cortex, on the growth of influenza A/PR/8 virus in vitro and in vivo.” Antiviral Research, vol. 74, 2007, pp. 1–8., doi:10.1016/j.antiviral.2007.01.003.
Jackson, William T. “Viruses and the autophagy pathway.” Virology, vol. 479-480, 2015, pp. 450-456. doi:10.1016/j.virol.2015.03.042
Kampf, Gunter, et al. “Efficacy of ethanol against viruses in hand disinfection.” Journal of Hospital Infection, 2017, pp. 170-178. doi:10.1016/j.jhin.2017.08.025
Kiyohara, Hiroaki, et al. “Patchouli alcohol: in vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth.” Journal of Natural Medicines, vol. 66, 2012, pp. 55-61. doi:10.1007/s11418-011-0550-x
Li, Runfeng, et al. “Pinanamine Is a Promising Lead Compound against Influenza A Virus: Evidence from in Vitro and in Vivo Efficacy Compared to Amantadine.” Biological and Pharmaceutical Bulletin, vol. 40, 2017, pp. 954-959.
Li, Xinghua, et al. “Meleleuca alternifolia Concentrate Inhibits in Vitro Entry of Influenza Virus into Host Cells.” Molecules, vol. 18, 2013, pp. 9550-9566. doi:10.3390/molecules18089550
Li, Yu-Cui, et al. “Oral administration of patchouli alcohol isolated from Pogostemonis Herba augments protection against influenza viral infection in mice.” International Immunopharmacology, vol. 12, 2012, pp. 294-301. doi:10.1016/j.intimp.2011.12.007
Liao, Qingjiao, et al. “Germacrone inhibits early stages of influenza virus infection.” Antiviral Research, vol. 100, 2013, pp. 578-588. doi:10.1016/j.antiviral.2013.09.021
Manzoor, Rashid, et al. “Influenza A Virus M2 Protein: Roles from Ingress to Egress.” International Journal of Molecular Sciences, vol. 18, no. 2649, 2017, pp. 1-16. doi:10.3390/ijms18122649
Matthews, T, Boehme, R. “Antiviral activity and mechanism of action of ganciclovir.” Reviews of Infectious Diseases, July 1988, pp. S490-494.
Nazzaro, F., et al. “Effect of essential oils on pathogenic bacteria.” Pharmaceuticals, vol. 6, no. 12, 2013, pp. 1451-1474., doi:10.3390/ph6121451
O’Grady, KF, et al. “Prevalence, codetection and seasonal distribution of upper airway viruses and bacteria in children with acute respiratory illnesses with cough as a symptom.” Clinical Microbiology and Infection, vol. 22, 2016, pp. 527-534. doi:10.1016/j.cmi.2016.02.004
Orhan, Ilkay Erdogan, et al. “Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Libiatae plants and individual essential oil components.” Turkish Journal of Biology, vol. 36, no. 3, 2012, pp. 239-246.
Paulpandi, Manickam, et al. “In vitro anti-viral effect of ꞵ-santalol against viral replication.” Phytomedicine, vol. 19, 2012, pp. 231-235. doi:10.1016/j.phymed.2011.11.006
Price, Shirley, Price, Len. Aromatherapy for Health Professionals. 3rd ed. 2007. Churchill Livingstone/Elsevier.
Pourghanbari, Gholamhosein, et al. “Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2).” Indian Journal of Virology, vol. 27, no. 2, 2016, pp. 170-178. doi:10.1007/s13337-016-0321-0
Samji, Tasleem. “Influenza A: Understanding the Viral Life Cycle.” Yale Journal of Biology and Medicine, vol. 82, 2009, pp. 153-159.
Setzer, William N. “Essential oils as complementary and alternative medicines for the treatment of influenza.” American Journal of Essential Oils and Natural Products, vol. 4, no. 4, 2016, pp. 16-22.
Shanks, GD. “Insights from unusual aspects of the 1918 influenza pandemic.” Travel Medicine and Disease, vol. 13, no. 3, 2015, pp. 217-222. doi:10.1016/j.tmaid.2015.05.001.
Tesini, Brenda L. “Influenza – Infectious Diseases.” Merck Manuals Professional Edition, June 2018, www.merckmanuals.com/professional/infectious-diseases/respiratory-viruses/influenza. Accessed September 25, 2018.
Tisoncik, Jennifer R., Korth, et al. “Into the eye of the cytokine storm.” Microbiology and Molecular Biology Reviews, vol. 76, no. 1, 2012, pp. 16-22., doi:10.1128/MMBR.05015-11
Tisserand, Robert. “Resistance is futile.” https://tisserandinstitute.org/resistance-is-futile/. 2015. Accessed December 11, 2018.
Tisserand, R., Young R., (2014) Essential oil safety: a guide for health care professionals, 2nd edition. London, Churchill Livingstone.
Tooze, SA, et al. “Endocytosis and Autophagy: Exploitation or Cooperation?” Cold Spring Harbor Perspectives in Biology, vol. 6, no. 5, 2014, pp. 1-15. doi:10.1101/cshperspect.a018358.
Usachev, Evgeny V, et al. “Antiviral activity of tea tree and eucalyptus oil aerosol and vapor.” Journal of Aerosol Science, vol. 59, 2013, pp. 22-30. doi:10.1016/j.jaerosci.2013.01.004
Vasconcelos, NG, et al. “Antibacterial mechanisms of cinnamon and its constituents: A review.” Microbial Pathogenesis, 2018, pp. 1-10. doi:10.1016/j.micpath.2018.04.036
Vimalanathan, Selvarani, and Hudson, James. “Anti-influenza virus activity of essential oils and vapors.” American Journal of Essential Oils and Natural Products, vol. 2, no. 1, 2014, pp. 47-53.
Wang, Yupeng, et al. “Autophagy in Negative-Strand RNA Virus Infection.” Frontiers in Microbiology, vol. 9, no. 206, 2018, pp. 1-11. doi:10.3389/fmicb.2018.00206
Wu, Huaxing, et al. “Inhibitory Effect and Possible Mechanisms of Action of Patchouli Alcohol against Influenza A (H3N2) Virus.” Journal of Ethnopharmacology, vol. 16, 2011, pp. 6489-6501. doi:10.3390/molecules16086489
Wu, Qiao-feng, et al. “Chemical compositions and anti-influenza activities of essential oils from Mosla dianthera.” Journal of Ethnopharmacology, vol. 139, 2012, pp. 668-671. doi:10.1016/j.jep.2011.11.056
Wu, Shuhua, et al. “Protective essential oil attenuates influenza virus infection: An in vitro study in MDCK cells.” BMC Complementary & Alternative Medicine, vol. 10, no. 69, 2010, pp. 1-13.
Zai-Chang, Yang, et al. “Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus.” Colloids and Surfaces B: Biointerfaces, vol. 43, 2005, pp. 198-202. doi:10.1016/j.colsurfb.2005.05.003
Zhao, Xin, et al. “Discovery of Highly Potent Pinanamine-Based Inhibitors against Amantadine- and Oseltamivir-Resistant Influenza A Viruses.” Journal of Medicinal Chemistry, vol. 61, 2018, pp. 5187-5198., doi:10.1021/acs.jmedchem.8b00042.
Zhang, Rong, et al. “The Regulation of Autophagy by Influenza A Virus.” BioMed Research International, vol. 2014, 2014, pp. 1-7. doi:10.1155/2014/498083



Very clearly written and informative article. It’s nice to see progress on the undervalued antiviral front, and hopefully more will come. There is also some in vivo research on 1,8-cineole that seems to be effective against influenza virus, either on its own or in combination with vaccine or Tamiflu. Also, it would be very interesting to see if there is indeed some specific, immune cell-activating “immunostimulatory” mechanism at work, as the study on patchoulol suggests, in addition to its anti-inflammatory action.
Petra, I agree, It’s tricky to talk about immunology in the context of aromatherapy, because it’s my opinion that it’s become a catch phrase. When looking at primary research, we can define it as anything that modulates mechanisms involved in the immune system, but those results don’t always equate to a clinical phenotype. I think there’s a little bit of a disconnect between the scientific definition of immunostimulatory versus “alternative medicine” or “healer” definitions. __Dr. Becker
This is an awesome article. Thank you for posting it at the perfect time.
Thank you Regina! –Dr. Becker
Really like the use of the research, charts, and diagrams. Thank you.
Thank you Cynthia! I am a visual learner, so I incorporate information more easily with figures and tables. It’s something I really like to do.
—Dr. Becker
Cleaning surfaces with chlorine bleach is highly dangerous as the fumes damage the lung tissues and is especially damaging for a person with a lung infection such as the flu.
There is a safer and much more affective surface cleaner named Thieves Concentrate household cleaner. It has been proven to kill the influenza virus whereas the bleach was not 100% effective.
Hi Vicki, I’d love to review this research you mention. The instructions for using bleach as a disinfectant is at 10% strength. Using undiluted bleach is unwise because of the fumes, and the skin irritation. However, it has been shown to be safe and effective at killing most microbials, including influenza, bacteria, and fungi. Some viruses, such as norovirus are more difficult to kill using alternative disinfectants, and bleach is really the best choice. Again, using as instructed. There is a lot of published, peer reviewed research showing its efficacy, and clinical research showing its use in medical settings.
I personally abhor the smell, so I don’t like using it. But, in some situations, I definitely use bleach.
My article is using evidence and peer review research to extrapolate usage advice, but again, this is based on primary research versus clinical application. Ideally in the future that will come, but medical settings MUST adhere to proven effective disinfectants, including bleach and other hospital grade substances.
I’m happy to link additional reading material, but “safe” is really about understanding proper use and evidence for efficacy. To my knowledge, Young Living has no data for this. Again, please feel free to share those. Until then, phrases like “it has been proven” are really not true. In addition, NOTHING is 100% effective for anything, including seat belts preventing injuries in car accidents, shampoos cleaning your hair, or indeed disinfection by bleach.
Thank you for publishing this information. So much to absorb but well worth taking the time to read. You’re right nothing is 100% but trying to minimize or avoid the flu would be great!
I like this study, I would like if you investigate also the influence of synergy. any way thank you Shannon Becker..
It’s hard to study at synergy of oils in vitro, other than comparing to specific constituents. In other words, comparing linalool, linalyl acetate, lavender EO.
Thank you so much for writing this articles.This article will cover a number of important points.Therefore,I highly recommend this article to anyone looking for such articles.
Thank you!
Hi
Thank you for the info! My question though is this, will wearing an antiviral mixture of oils be as effective (or effective enough) as diffusing it? You cant always carry a diffuser every where you go, even the handheld kind. So will applying a mixture periodically through the day be helpful?
Thanks!
Hi! The recommendations in the article were based on in vitro and in vivo research, and we don’t know if they definitively will work in human clinical use, unfortunately. However, I think it’s reasonable to make these suggestions. I think having a diffuser necklace would be comparable to a sniffy stick.
Shannon, thanks for a great article. Regarding the rosemary mentioned several times, any indication of chemotype? Thanks!
It’s the 1,8-cineole chemotype.
I have found this article a great help and have used the virus cycle illustrations in particular to select oils, however, where a chemical constituent of an essential oil is named it would perhaps be helpful to some of us to have a list of these constituents and which oil they are in at the bottom of the article as I have no idea which has rimantadine etc. Also, I am not clear whether all the processes in the cycle of virus replication are one time events or continually recurring so I’m not sure whether to stop using , for example, tea tree oil if I’ve already got flu and to start on the lemongrass etc or whether you should use a drop of each all the time to cover everything. A bit confused! Thanks for such a clearly set out, accessible article.
Thank you!
Cells in your body are constantly going through their reproductive cycles, and they are not synchronized. So if you think of the liver cells, they aren’t waiting on each other at the starting line of a race. They are constantly reacting to their environmental cues, and they affect their cell cycle. Although viruses are not alive, they do have some characteristics in common with cells. They use the cellular machinery to reproduce and spread. Viruses in a body are not synchronized either. The figures are like a snap shot showing all of the steps, so it’s not possible to say “ok, the viruses in my body is doing this right now”, except on general terms. For influenza, the timeline of infection and symptoms is well studied, so they can say “early stage”, etc. Hope that helps! -Dr. Becker
One of the best articles out there if you’re considering oils for sanitation, Great writing style, thanks.
I wonder, based on your knowledge of influenza, do you think it’s it reasonable that the oils might also break down COVID-19 ?
I have a further question if possible, regarding clove bud oil, your article states syzygium aromaticum for clove bud oil but if the only thing you can find is the leaf oil for that or otherwise the bud for a different type of clove-eugenia caryophyllata, which would be the best of those two to go for? Also, with regard to cinnamomum cassia, I am looking for this at the moment and wondered if it is any particular part- leaf, bark etc, or just cinnamomum cassia oil and the part of the plant doesn’t matter. Thank you, I find more in this article every time I read it!
WOW , AWESOME INFO.LEARNED SOMETHING NEW.
THANXZ
This is an Awesome Article and A lot of Research. Thank You for Sharing!
Hello and thank you for the article. Can you tell me please what is an Inhaler as reffered to in the below statement.
“For the home user, an inhaler containing anti-influenza essential oils would be another option.”
Greetings, I will attach a link for you to a trusted AromaWeb article that has great information on Aromatherapy Inhalers. 🙂
https://www.aromaweb.com/articles/aromatherapyinhalers.asp
All the best, Shane Carper